Senecionine
 | |
| Names | |
|---|---|
| Other names
Aureine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.125.118 |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C18H25NO5 | |
| Density | 1.25 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Senecionine is an organic compound with the chemical formula C
18H
25NO
5. It is produced by Senecio species and classified as a pyrrolizidine alkaloid. It has a core structure of retronecine which is further esterified by two Isoleucine to form a 12 member lactone ring. The compound is toxic and as alkaloid of the widespread plant Jacobaea vulgaris also problematic for feed stock and human consumption.[1]
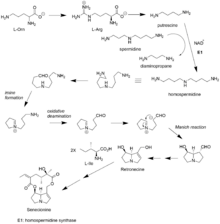
Biosynthesis
In Senecio species, biosynthesis of Senecionine starts from L-Arginine or L-ornithine. Since plants don't have decarboxylase enzyme for L-ornithine, it must be first converted into L-Arginine, which can be readily converted to putrescine. Putrescine gains an aminopropyl group from spermidine in an NAD+ fashion to form homospermidine via homospermidine synthase. The detailed mechanism for this step isn't well understood and it is suspected that imine intermediate is involved. Homospermidine undergo a series of reactions to form the 2 five member rings structure of retronecine. Retronecine is further esterified by two L-isoleucine and modified through a couple of steps to senecionine.
See also
- Riddelliine, a closely related pyrrolizidine alkaloid
References
- ↑ "Pyrrolizidine Alkaloids—Genotoxicity, Metabolism Enzymes, Metabolic Activation, and Mechanisms". doi:10.1081/DMR-120028426.
- Dewick, M,Paul (Feb 4, 2009). Medicinal Natural Products. wiley online. pp. 324–325. doi:10.1002/9780470742761. ISBN 9780470742761.