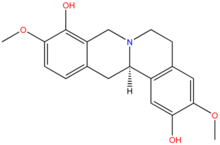
Scoulerine
 | |
| Names | |
|---|---|
| IUPAC name
(13aS)-3,10-dimethoxy-5,8,13,13a- | |
| Other names
Discretamine, Aequaline, Scoulerin. | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C19H21NO4 | |
| Molar mass | 327.37 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Scoulerine, also known as discretamine and aequaline, is a benzylisoquinoline alkaloid that derives from reticuline and is a precursor of berberine. It is found in many plants, including Opium poppy,[1] Croton flavens,[2] and certain plants in the Erythrina genus.[3]
Studies show that scoulerine is an antagonist in vitro at the α2-adrenoceptor, α1D-adrenoceptor and 5-HT receptor.[4][5] It has also been found to be a GABAA receptor agonist in vitro.[2][6]
References
- ↑ Frick S; Chitty JA; Kramell R; Schmidt J; Allen RS; Larkin PJ; Kutchan TM (2004). "Transformation of opium poppy (Papaver somniferum L.) with antisense berberine bridge enzyme gene (anti-bbe) via somatic embryogenesis results in an altered ratio of alkaloids in latex but not in roots". Transgenic Res. 13 (6): 607&ndash, 613. doi:10.1007/s11248-004-2892-6. PMID 15672841.
- 1 2 Eisenreich WJ; Hofner G; Bracher F (2003). "Alkaloids from Croton flavens L. and their affinities to GABA-receptors". Nat Prod Res. 17 (6): 437&ndash, 440. doi:10.1080/1478641031000111516. PMID 14577695.
- ↑ Ito K (1999). "Studies on the alkaloids of Erythrina plants". Yakugaku Zasshi. 119 (5): 340&ndash, 356. PMID 10375996.
- ↑ Ko FN; Yu SM; Su MJ; Wu YC; Teng CM (1993). "Pharmacological activity of (−)-discretamine, a novel vascular α-adrenoceptor and 5-hydroxytryptamine receptor antagonist, isolated from Fissistigma glaucescens". Br J Pharmacol. 110 (2): 882&ndash, 888. doi:10.1111/j.1476-5381.1993.tb13895.x. PMC 2175899. PMID 7902181.
- ↑ Ko FN; Guh JH; Yu SM; Hou YS; Wu YC; Teng CM (1994). "(−)-Discretamine, a selective α1D-adrenoceptor antagonist, isolated from Fissistigma glaucescens". Br J Pharmacol. 112 (4): 1174&ndash, 1180. doi:10.1111/j.1476-5381.1994.tb13207.x. PMC 1910235. PMID 7952879.
- ↑ Halbsguth C; Meissner O; Haberlein H (2003). "Positive cooperation of protoberberine type 2 alkaloids from Corydalis cava on the GABA(A) binding site". Planta Med. 69 (4): 305&ndash, 309. doi:10.1055/s-2003-38869. PMID 12709895.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.