Schottky defect
A Schottky defect is a type of point defect in a crystal lattice named after Walter H. Schottky. In non-ionic crystals it means a lattice vacancy defect.
In ionic crystals, the defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid. The surrounding atoms then move to fill these vacancies, causing new vacancies to form. Normally these defects will lead to a decrease in the density of the crystal. The following are the chemical equations in Kröger–Vink notation for the formation of Schottky defects in TiO2 and BaTiO3.
- ∅ ⇌ v′′′′
Ti + 2 v••
O
- ∅ ⇌ v′′
Ba + v′′′′
Ti + 3 v••
O
This can be illustrated schematically with a two-dimensional diagram of a sodium chloride crystal lattice:
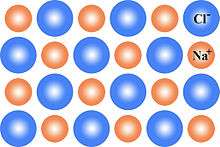

Definition
Schottky defects consists of unoccupied anion and cation sites in a stoichiometric ratio. For a simple ionic crystal of type A−B+, a Schottky defect consists of a single anion vacancy (A) and a single cation vacancy (B), or v•
A + v′
B following Kröger–Vink notation. For a more general crystal with formula AxBy, a Schottky cluster is formed of x vacancies of A and y vacancies of B, thus the overall stoichiometry and charge neutrality are conserved.
Schottky defects are observed most frequently when there is a small difference in size between cations and anions. This is produced as the result of the thermal incorporation of unoccupied lattice sites from the exterior of the crystal. The lattice undergoes thermal vibration and thermal expansion when the temperature is raised above 0 K. When it happens the pair of vacancies are incorporated in the crystal. So electrical neutrality is maintained inside the crystals.
Bound and dilute defects

The vacancies that make up the Schottky defects have opposite charge, thus they experience a mutually attractive Coulomb force. If sufficient thermal energy is available the vacancies may migrate through the crystal lattice, and form bound clusters.
The bound clusters are typically less mobile than the dilute counterparts, as multiple species need to move in a concerted motion for the whole cluster to migrate. This has important implications for numerous functional ceramics used in a wide range of applications, including ion conductors, Solid oxide fuel cells and nuclear fuel.[1]
Examples
This type of defect is typically observed in highly ionic compounds, highly coordinated compounds, and where there is only a small difference in sizes of cations and anions of which the compound lattice is composed. Typical salts where Schottky disorder is observed are NaCl, KCl, KBr, CsCl and AgBr. For engineering applications, Schottky defects are important in oxides with Fluorite structure, such as CeO2, cubic ZrO2, UO2, ThO2 and PuO2.
Effect on density
Since the total number of ions present in the crystal with this defect is less than the theoretical number of ions for a crystal of its volume, the density of the solid crystal is less than the theoretical density of the material.
See also
References
- 1 2 Burr, P. A.; Cooper, M. W. D. (2017-09-15). "Importance of elastic finite-size effects: Neutral defects in ionic compounds". Physical Review B. 96 (9): 094107. arXiv:1709.02037. Bibcode:2017PhRvB..96i4107B. doi:10.1103/PhysRevB.96.094107.
- Kittel, Charles (2005). Introduction to Solid State Physics (8th ed.). Wiley. ISBN 978-0-471-41526-8.