SENP2
Sentrin-specific protease 2 is an enzyme that in humans is encoded by the SENP2 gene.[5][6][7]
Function
SUMO1 (UBL1; MIM 601912) is a small ubiquitin-like protein that can be covalently conjugated to other proteins. SENP2 is one of a group of enzymes that process newly synthesized SUMO1 into the conjugatable form and catalyze the deconjugation of SUMO1-containing species.[supplied by OMIM][7]
Interactions
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000163904 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000022855 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 Hang J, Dasso M (May 2002). "Association of the human SUMO-1 protease SENP2 with the nuclear pore". J. Biol. Chem. 277 (22): 19961–6. doi:10.1074/jbc.M201799200. PMID 11896061.
- ↑ Nishida T, Kaneko F, Kitagawa M, Yasuda H (October 2001). "Characterization of a novel mammalian SUMO-1/Smt3-specific isopeptidase, a homologue of rat axam, which is an axin-binding protein promoting beta-catenin degradation". J. Biol. Chem. 276 (42): 39060–6. doi:10.1074/jbc.M103955200. PMID 11489887.
- 1 2 "Entrez Gene: SENP2 SUMO1/sentrin/SMT3 specific peptidase 2".
- ↑ Zhang H, Saitoh H, Matunis MJ (September 2002). "Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex". Mol. Cell. Biol. 22 (18): 6498–508. doi:10.1128/MCB.22.18.6498-6508.2002. PMC 135644. PMID 12192048.
Further reading
- Mikolajczyk J, Drag M, Békés M, Cao JT, Ronai Z, Salvesen GS (2007). "Small ubiquitin-related modifier (SUMO)-specific proteases: profiling the specificities and activities of human SENPs". J. Biol. Chem. 282 (36): 26217–24. doi:10.1074/jbc.M702444200. PMID 17591783.
- Drag M, Mikolajczyk J, Krishnakumar IM, Huang Z, Salvesen GS (2008). "Activity profiling of human deSUMOylating enzymes (SENPs) with synthetic substrates suggests an unexpected specificity of two newly characterized members of the family". Biochem. J. 409 (2): 461–9. doi:10.1042/BJ20070940. PMID 17916063.
- Nakajima D, Okazaki N, Yamakawa H, Kikuno R, Ohara O, Nagase T (2002). "Construction of expression-ready cDNA clones for KIAA genes: manual curation of 330 KIAA cDNA clones". DNA Res. 9 (3): 99–106. doi:10.1093/dnares/9.3.99. PMID 12168954.
- Nagase T, Kikuno R, Ishikawa KI, Hirosawa M, Ohara O (2000). "Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro". DNA Res. 7 (1): 65–73. doi:10.1093/dnares/7.1.65. PMID 10718198.
- Kadoya T, Yamamoto H, Suzuki T, Yukita A, Fukui A, Michiue T, Asahara T, Tanaka K, Asashima M, Kikuchi A (2002). "Desumoylation activity of Axam, a novel Axin-binding protein, is involved in downregulation of beta-catenin". Mol. Cell. Biol. 22 (11): 3803–19. doi:10.1128/MCB.22.11.3803-3819.2002. PMC 133821. PMID 11997515.
- Zhang H, Saitoh H, Matunis MJ (2002). "Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex". Mol. Cell. Biol. 22 (18): 6498–508. doi:10.1128/MCB.22.18.6498-6508.2002. PMC 135644. PMID 12192048.
- Best JL, Ganiatsas S, Agarwal S, Changou A, Salomoni P, Shirihai O, Meluh PB, Pandolfi PP, Zon LI (2002). "SUMO-1 protease-1 regulates gene transcription through PML". Mol. Cell. 10 (4): 843–55. doi:10.1016/S1097-2765(02)00699-8. PMID 12419228.
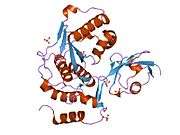
- Reverter D, Lima CD (2004). "A basis for SUMO protease specificity provided by analysis of human Senp2 and a Senp2-SUMO complex". Structure. 12 (8): 1519–31. doi:10.1016/j.str.2004.05.023. PMID 15296745.
- Itahana Y, Yeh ET, Zhang Y (2006). "Nucleocytoplasmic shuttling modulates activity and ubiquitination-dependent turnover of SUMO-specific protease 2". Mol. Cell. Biol. 26 (12): 4675–89. doi:10.1128/MCB.01830-05. PMC 1489137. PMID 16738331.
- Reverter D, Lima CD (2006). "Structural basis for SENP2 protease interactions with SUMO precursors and conjugated substrates". Nat. Struct. Mol. Biol. 13 (12): 1060–8. doi:10.1038/nsmb1168. PMID 17099700.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.