Rubber elasticity
Rubber elasticity, a well-known example of hyperelasticity, describes the mechanical behavior of many polymers, especially those with cross-links.
History
Following its introduction to Europe from the New World in the late 15th century, rubber was regarded mostly as a fascinating curiosity. Its most useful application was its ability to erase pencil marks on paper by rubbing, hence its name. It was not until 1838 that the American inventor Charles Goodyear found that its properties could be immensely improved by adding a few percent sulphur. The short sulfur chains produced chemical crosslinks. Before it is crosslinked, the liquid natural rubber consists of very long linear chains, containing thousands of isoprene backbone units, connected head-to-tail. Every chain follows a random path through the liquid and is in contact with thousands of other nearby chains. When heated, a crosslinker molecule (such as sulfur or dicumyl peroxide) can create a chemical bond (a network node) between two adjacent chains in the liquid, resulting in a three dimensional network. All of the original separate linear chains are connected together at multiple points to form a single giant molecule. The sections between two crosslinks on the same chain are called network chains and can contain up to several hundred isoprene units. In natural rubber, each crosslink produces a network node with four chains emanating from it. The network is the sine qua non of elastomers. Because of the enormous economic and technological importance of rubber, determining how a molecular network responds to mechanical strains has been of enduring interest to scientists and engineers. To understand the elastic properties of rubber, theoretically, it is necessary to know both the physical mechanisms that occur at the molecular level and how the random-walk nature of the chain produces the network. The physical mechanisms that occur within short sections of the polymer chains produce the elastic forces and the network morphology determines how these forces combine to produce the macroscopic stress that we observe when a rubber sample is deformed, e.g. subjected to tensile strain.
Molecular-level models
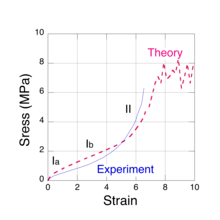
There are actually several physical mechanisms that produce the elastic forces within the network chains as a rubber sample is stretched. Two of these arise from entropy changes and one is associated with the distortion of the molecular bond angles along the chain backbone. These three mechanisms are immediately apparent when a moderately thick rubber sample is stretched manually. Initially, the rubber feels quite stiff, i.e. the force must be increased at a high rate with respect to the strain. At intermediate strains, the required increase in force is much lower to cause the same amount of stretch. Finally, as the sample approaches the breaking point, its stiffness increases markedly. What the observer is noticing are the changes in the modulus of elasticity that are due to the different molecular mechanisms. These regions can be seen in Fig. 1, a typical stress vs. strain measurement for natural rubber. The three mechanisms (labelled Ia, Ib and II) predominantly correspond to the regions shown on the plot. The concept of entropy comes to us from the area mathematical physics called Statistical mechanics which is concerned with the study of large thermal systems, e.g. rubber networks at room temperature. Although the detailed behavior of the constituent chains are random and far too complex to study individually, we can obtain very useful information about their 'average' behavior from a statistical mechanics analysis of a large sample. There are no other examples of how entropy changes can produce a force in our everyday experience. One may regard the entropic forces in polymer chains as arising from the thermal collisions that their constituent atoms experience with the surrounding material. It is this constant jostling that produces a resisting (elastic) force in the chains as they are forced to become straight.
When these elastic force models are combined with the complex morphology of the network, it is not possible to obtain simple analytic formulae to predict the macroscopic stress. It is only via numerical simulations on computers that it is possible to capture the complex interaction between the molecular forces and the network morphology to predict the stress and ultimate failure of a rubber sample as it is strained.
The Molecular Kink Paradigm for rubber elasticity[1]
The Molecular Kink Paradigm proceeds from the intuitive notion that molecular chains that make up a natural rubber (polyisoprene) network are constrained by surrounding chains to remain within a ‘tube’. Elastic forces produced in a chain, as a result of some applied strain, are propagated along the chain contour within this tube. Fig. 2 shows a representation of a four-carbon isoprene backbone unit with an extra carbon atom at each end to indicate its connections to adjacent units on a chain. It has three single C-C bonds and one double bond. It is principally by rotating about the C-C single bonds that a polyisoprene chain randomly explores its possible conformations. Sections of chain containing between two and three isoprene units have sufficient flexibility that they may be considered statistically de-correlated from one another. That is, there is no directional correlation along the chain for distances greater than this distance, referred to as a Kuhn length. These non-straight regions evoke the concept of ‘kinks’ and are in fact a manifestation of the random-walk nature of the chain. Since a kink is composed of several isoprene units, each having three carbon-carbon single bonds, there are many possible conformations available to a kink, each with a distinct energy and end-to-end distance. Over time scales of seconds to minutes, only these relatively short sections of the chain, i.e. kinks, have sufficient volume to move freely amongst their possible rotational conformations. The thermal interactions tend to keep the kinks in a state of constant flux, as they make transitions between all of their possible rotational conformations. Because the kinks are in thermal equilibrium, the probability that a kink resides in any rotational conformation is given by a Boltzmann distribution and we may associate an entropy with its end-to-end distance. The probability distribution for the end-to-end distance of the kink is approximately Gaussian and is determined by the Boltzmann probability factors for each state. As a rubber network is stretched, some kinks are forced into a restricted number of more extended conformations (greater end-to-end distance) and it is the resulting decrease in entropy that produces an elastic force along the chain.
There are three distinct molecular mechanisms that produce these forces, two of which arise from changes in entropy that we shall refer to as low chain extension regime, Ia [2] and moderate chain extension regime, Ib.[3] The third mechanism occurs at high chain extension, as it is extended beyond its initial equilibrium contour length by the distortion of the chemical bonds along its backbone. In this case, the restoring force is spring-like and we shall refer to it as regime II.[4] The three force mechanisms are found to roughly correspond to the three regions observed in tensile stress vs. strain experiments, shown in Fig. 1.
The initial morphology of the network, immediately after chemical cross-linking, is governed by two random processes:[5][6] (1) The probability for a cross-link to occur at any isoprene unit and, (2) the random walk nature of the chain conformation. The end-to-end distance probability distribution for a fixed chain length, i.e. fixed number of isoprene units, is described by a random walk. It is the joint probability distribution of the network chain lengths and the end-to-end distances between their cross-link nodes that characterizes the network morphology. Because both the molecular physics mechanisms that produce the elastic forces and the complex morphology of the network must be treated simultaneously, simple analytic elasticity models are not possible; an explicit 3-dimensional numerical model[7][8][9] is required to simulate the effects of strain on a representative volume element of a network.
Low chain extension regime, Ia
The Molecular Kink Paradigm envisions a representative network chain as a series of vectors that follow the chain contour within its tube. Each vector represents the equilibrium end-to-end distance of a kink. The actual 3-dimensional path of the chain is not pertinent, since all elastic forces are assumed to operate along the chain contour. In addition to the chain’s contour length, the only other important parameter is its tortuosity, the ratio of its contour length to its end-to-end distance. As the chain is extended, in response to an applied strain, the induced elastic force is assumed to propagate uniformly along its contour. Consider a network chain whose end points (network nodes) are more or less aligned with the tensile strain axis. As the initial strain is applied to the rubber sample, the network nodes at the ends of the chain begin to move apart and all of the kink vectors along the contour are stretched simultaneously. Physically, the applied strain forces the kinks to stretch beyond their thermal equilibrium end-to-end distances, causing a decrease in their entropy. The increase in free energy associated with this change in entropy, gives rise to a (linear) elastic force that opposes the strain. The force constant for the low strain regime can be estimated by sampling molecular dynamics (MD) trajectories of a kink, i.e. short chains, composed of 2-3 isoprene units, at relevant temperatures, e.g. 300K.[2] By taking many samples of the coordinates over the course of the simulations, the probability distributions of end-to-end distance for a kink can be obtained. Since these distributions (which turn out to be approximately Gaussian) are directly related to the number of states, we may associate them with the entropy of the kink at any end-to-end distance. By numerically differentiating the probability distribution, the change in entropy, and hence free energy, with respect to the kink end-to-end distance can be found. The force model for this regime is found to be linear and proportional to the temperature divided by the chain tortuosity.

Moderate chain extension regime, Ib
At some point in the low extension regime, i.e. as all of the kinks along the chain are being extended simultaneously, it becomes energetically more favorable to have one kink transition to an extended conformation in order to stretch the chain further. The applied strain can force a single isoprene unit within a kink into an extended conformation, slightly increasing the end-to-end distance of the chain, and the energy required to do this is less than that needed to continue extending all of the kinks simultaneously. Numerous experiments [10] strongly suggest that stretching a rubber network is accompanied by a decrease in entropy. As shown in Fig. 2, an isoprene unit has three single C-C bonds and there are two or three preferred rotational angles (orientations) about these bonds that have energy minima. Of the18 allowed[3] rotational conformations, only 6 have extended end-to-end distances and forcing the isoprene units in a chain to reside in some subset of the extended states must reduce the number of rotational conformations available for thermal motion. It is this reduction in the number of available states that causes the entropy to decrease. As the chain continues to straighten, all of the isoprene units in the chain are eventually forced into extended conformations and the chain is considered to be ‘taut’. A force constant for chain extension can be estimated from the resulting change in free energy associated with this entropy change.[3] As with regime Ia, the force model for this regime is linear and proportional to the temperature divided by the chain tortuosity.
High chain extension regime, II
When all of the isoprene units in a network chain have been forced to reside in just a few extended rotational conformations, the chain is sensibly straight, except for the zigzag path that the C-C bonds make along the chain contour. However, further extension is still possible by bond distortions, e.g., bond angle increases, bond stretches and dihedral angle rotations. These forces are spring-like and are not associated with entropy changes. At an extension of about 40%, the tensile force along the chain is sufficient to mechanically rupture one of the C-C covalent bonds. The tensile force for bond rupture has been calculated[4] via quantum chemistry simulations and it is approximately 7 nN, about a factor of a thousand greater than the entropic chain forces at low strain. The angles between adjacent backbone C-C bonds in an isoprene unit vary between about 115-120 degrees and the forces associated with maintaining these angles are quite large, so within each unit, the chain backbone always follows a zigzag path, even at bond rupture. This mechanism accounts for the steep upturn in the elastic stress, observed at high strains (Fig. 1).
Network morphology
Although the network is completely described by only two parameters (the number of network nodes per unit volume and the statistical de-correlation length of the polymer), the way in which the chains are connected is quite complicated. There is a wide variation in the lengths of the chains and most of them are not connected to the nearest neighbor network node. Both the chain length and its end-to-end distance are described by probability distributions. The term ‘morphology’ refers to this complexity. If the crosslinking agent is thoroughly mixed, there is an equal probability for any isoprene unit to become a network node. For dicumyl peroxide, the cross linking efficiency in natural rubber is unity, but this is not the case for sulfur.[11] The initial morphology of the network is dictated by two random processes: the probability for a crosslink to occur at any isoprene unit and the Markov random walk nature of a chain conformation.[5][6] The probability distribution function for how far one end of a chain end can ‘wander’ from the other is generated by a Markov sequence.[12] This conditional probability density function relates the chain length in units of the Kuhn length to the end-to-end distance :
-
(1)
The probability that any isoprene unit becomes part of a cross-link node is proportional to the ratio of the concentrations of the cross-linker molecules (e.g., dicumyl-peroxide) to the isoprene units:
-
(2)
The factor of two comes about because two isoprene units (one from each chain) participate in the crosslink. The probability for finding a chain containing isoprene units is given by:
-
(3)
where . The equation can be understood as simply the probability that an isoprene unit is NOT a crosslink (1-px) in N-1 successive units along a chain. Since P(N) decreases with N, shorter chains are more probable than longer ones. Note that the number of statistically independent backbone segments is not the same as the number of isoprene units. For natural rubber networks, the Kuhn length contains about 2.2 isoprene units, so . It is the product of equations (1) and (3) (the joint probability distribution) that relates the network chain length ( ) and end-to-end distance ( ) between its terminating cross-link nodes:
-
(4)
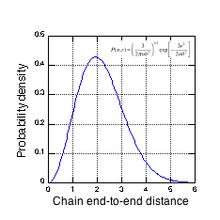
The complex morphology of a natural rubber network can be seen in Fig. 3, which shows the probability density vs. end-to-end distance (in units of mean node spacing) for an ‘average’ chain. For the common experimental cross-link density of 4x1019 cm−3, an average chain contains about 116 isoprene units (52 Kuhn lengths) and has a contour length of about 50 nm. Fig. 3 shows that a significant fraction of chains span several node spacings, i.e., the chain ends overlap other network chains. Natural rubber, crosslinked with dicumyl peroxide, has tetra-functional crosslinks, i.e. each crosslink node has 4 network chains emanating from it. Depending on their initial tortuosity and the orientation of their endpoints with respect to the strain axis, each chain associated with an active crosslink node can have a different elastic force constant as it resists the applied strain. To preserve force equilibrium (zero net force) on each crosslink node, a node may be forced to move in tandem with the chain having the highest force constant for chain extension. It is this complex node motion, arising from the random nature of the network morphology, that makes the study of the mechanical properties of rubber networks so difficult. As the network is strained, paths composed of these more extended chains emerge that span the entire sample, and it is these paths that carry most of the stress at high strains.
Numerical network simulation model
To calculate the elastic response of a rubber sample, the three chain force models (regimes Ia, Ib and II) and the network morphology must be combined in a micro-mechanical network model.[7][8][9] Using the joint probability distribution in equation (4) and the force extension models, it is possible to devise numerical algorithms to both construct a faithful representative volume element of a network and to simulate the resulting mechanical stress as it is subjected to strain. An iterative relaxation algorithm is used to maintain approximate force equilibrium at each network node as strain is imposed. When the force constant obtained for kinks having 2 or 3 isoprene units (approximately one Kuhn length) is used in numerical simulations, the predicted stress is found to be consistent with experiments. The results of such a calculation[11] are shown in Fig. 1 (dashed red line) for sulfur cross-linked natural rubber and compared with experimental data[13] (solid blue line). These simulations also predict a steep upturn in the stress as network chains become taut and, ultimately, material failure due to bond rupture. In the case of sulfur cross-linked natural rubber, the S-S bonds in the crosslink are much weaker than the C-C bonds on the chain backbone and are the network failure points. The plateau in the simulated stress, starting at a strain of about 7, is the limiting value for the network. Stresses greater than about 7 MPa cannot be supported and the network fails. Near this stress limit, the simulations predict[9] that less than 10% of the chains are taut, i.e. in the high chain extension regime and less than 0.1% of the chains have ruptured. While the very low rupture fraction may seem surprising, it is not inconsistent with our experience of stretching a rubber band until it breaks. The elastic response of the rubber after breaking is not noticeably different from the original.
Historical approaches to elasticity theory
Eugene Guth and Hubert M. James proposed the entropic origins of rubber elasticity in 1941.[14]
Thermodynamics
Temperature affects the elasticity of elastomers in an unusual way. When the elastomer is assumed to be in a stretched state, heating causes them to contract. Vice versa, cooling can cause expansion.[15] This can be observed with an ordinary rubber band. Stretching a rubber band will cause it to release heat (press it against your lips), while releasing it after it has been stretched will lead it to absorb heat, causing its surroundings to become cooler. This phenomenon can be explained with the Gibbs Free Energy. Rearranging ΔG=ΔH−TΔS, where G is the free energy, H is the enthalpy, and S is the entropy, we get TΔS=ΔH−ΔG. Since stretching is nonspontaneous, as it requires external work, TΔS must be negative. Since T is always positive (it can never reach absolute zero), the ΔS must be negative, implying that the rubber in its natural state is more entangled (with more microstates) than when it is under tension. Thus, when the tension is removed, the reaction is spontaneous, leading ΔG to be negative. Consequently, the cooling effect must result in a positive ΔH, so ΔS will be positive there.[16][17]
The result is that an elastomer behaves somewhat like an ideal monatomic gas, inasmuch as (to good approximation) elastic polymers do not store any potential energy in stretched chemical bonds or elastic work done in stretching molecules, when work is done upon them. Instead, all work done on the rubber is "released" (not stored) and appears immediately in the polymer as thermal energy. In the same way, all work that the elastic does on the surroundings results in the disappearance of thermal energy in order to do the work (the elastic band grows cooler, like an expanding gas). This last phenomenon is the critical clue that the ability of an elastomer to do work depends (as with an ideal gas) only on entropy-change considerations, and not on any stored (i.e., potential) energy within the polymer bonds. Instead, the energy to do work comes entirely from thermal energy, and (as in the case of an expanding ideal gas) only the positive entropy change of the polymer allows its internal thermal energy to be converted efficiently (100% in theory) into work.
Polymer chain theories
Invoking the theory of rubber elasticity, one considers a polymer chain in a crosslinked network as an entropic spring. When the chain is stretched, the entropy is reduced by a large margin because there are fewer conformations available.[18] Therefore, there is a restoring force, which causes the polymer chain to return to its equilibrium or unstretched state, such as a high entropy random coil configuration, once the external force is removed. This is the reason why rubber bands return to their original state. Two common models for rubber elasticity are the freely-jointed chain model and the worm-like chain model.
Freely-jointed chain model
Polymers can be modeled as freely jointed chains with one fixed end and one free end (FJC model):
where is the length of a rigid segment, is the number of segments of length , is the distance between the fixed and free ends, and is the "contour length" or . Above the glass transition temperature, the polymer chain oscillates and changes over time. The probability of finding the chain ends a distance apart is given by the following Gaussian distribution:
Note that the movement could be backwards or forwards, so the net time average will be zero. However, one can use the root mean square as a useful measure of that distance.
Ideally, the polymer chain's movement is purely entropic (no enthalpic, or heat-related, forces involved). By using the following basic equations for entropy and Helmholtz free energy, we can model the driving force of entropy "pulling" the polymer into an unstretched conformation. Note that the force equation resembles that of a spring: F=kx.
Note that the elastic coefficient is temperature dependent. If we increase the rubber temperature, the elastic coefficient also rises. This is the reason why rubber under constant stress shrinks when its temperature increases.
Worm-like chain model
The worm-like chain model (WLC) takes the energy required to bend a molecule into account. The variables are the same except that , the persistence length, replaces . Then, the force follows this equation:
Therefore, when there is no distance between chain ends (r=0), the force required to do so is zero, and to fully extend the polymer chain ( ), an infinite force is required, which is intuitive. Graphically, the force begins at the origin and initially increases linearly with . The force then plateaus but eventually increases again and approaches infinity as the chain length approaches
See also
References
- ↑ D. E. Hanson and J. L. Barber, Contemporary Physics 56 (3), 319-337 (2015)
- 1 2 D. E. Hanson and R. L. Martin, Journal of Chemical Physics 133, 084903 (084908 pp.) (2010)
- 1 2 3 D. E. Hanson, J. L. Barber and G. Subramanian, Journal of Chemical Physics 139 (2013)
- 1 2 D. E. Hanson and R. L. Martin, The Journal of Chemical Physics 130, 064903 (2009)
- 1 2 P. Flory, N. Rabjohn and M. Shaffer, Journal of Polymer Science 4, 435-455 (1949)
- 1 2 D. E. Hanson, Journal of Chemical Physics 134, 064906 (064906 pp.) (2011)
- 1 2 D. E. Hanson, Polymer 45 (3), 1058-1062 (2004)
- 1 2 D. E. Hanson, Journal of Chemical Physics 131, 224904 (224905 pp.) (2009)
- 1 2 3 D. E. Hanson and J. L. Barber, Modelling and Simulation in Materials Science and Engineering 21 (2013)
- ↑ J. P. Joule, Phil. Trans. R. Soc. London 149, 91–131 (1859)
- 1 2 D. E. Hanson and J. L. Barber, Phys. Chem. Chem. Phys. 20, 8460 (2018)
- ↑ A. A. Markov, Izv. Peterb. Akad. 4 (1), 61-80 (1907)
- ↑ L. R. G. Treloar, Trans. Faraday Soc., 40, 0059 (1944)
- ↑ Guth, Eugene; James, Hubert M. (May 1941). "Elastic and Thermoelastic Properties of Rubber like Materials". Ind. Eng. Chem. 33 (5): 624–629. doi:10.1021/ie50377a017.
- ↑ "Thermodynamics of a Rubber Band", American Journal of Physics, 31 (5): 397&ndash, 397, May 1963, Bibcode:1963AmJPh..31..397T, doi:10.1119/1.1969535
- ↑ Rubber Bands and Heat, http://scifun.chem.wisc.edu/HomeExpts/rubberband.html, citing Shakhashiri (1983)
- ↑ Shakhashiri, Bassam Z. (1983), Chemical Demonstrations: A Handbook for Teachers of Chemistry, 1, Madison, WI: The University of Wisconsin Press, ISBN 978-0-299-08890-3
- ↑ L.R.G. Treloar (1975), Physics of Rubber Elasticity, Oxford University Press, ISBN 9780198570271