Vagal tone
Vagal tone refers to activity of the vagus nerve, a fundamental component of the parasympathetic branch of the autonomic nervous system. This branch of the nervous system is not under conscious control and is largely responsible for the regulation of several body compartments at rest. Vagal activity results in various effects, including: heart rate reduction, vasodilation/constriction of vessels, glandular activity in the heart, lungs, and digestive tract as well as control of gastrointestinal sensitivity, motility and inflammation.[1]
In this context, tone specifically refers to the continual nature of baseline parasympathetic action that the vagus nerve exerts. While baseline vagal input is constant, the degree of stimulation it exerts is regulated by a balance of inputs from sympathetic and parasympathetic divisions of the autonomic nervous system. Despite the described duality, vagal tone has been reported to mainly reflects the general level of parasympathetic activity. Vagal tone is typically considered in the context of heart function, but also has utility in assessing emotional regulation and other processes that alter, or are altered by changes and modification of the parasympathetic activity.[2][3]
Measuring vagal tone along with its quantification and estimation can be performed by means of either invasive or noninvasive procedures. The former methodologies encompass the vagus nerve stimulation by manual or electrical techniques but literature reports a very limited number of experiments and clinical studies especially involving human subjects. On the other hand, noninvasive techniques are largely employed and they mainly rely on the investigation of heart rate and heart rate variability.[4][5][6]
Noninvasive vagal tone quantification
In the majority of cases, vagal tone is not directly measured. The most common procedure towards its quantification consist in investigating the processes altered by the vagus nerve - specifically heart rate and heart rate variability. As a general consideration, increased vagal tone (and thus vagal action) is associated with a diminished and more variable heart rate. On the opposite, during graded orthostatic tilt, vagal tone withdrawal is physiological and described as an indirect indicator of cardiovascular fitness.[7]
Vagal innervation of the heart
Heart rate is largely controlled by the heart's internal pacemaker activity. Considering a healthy heart, the main pacemaker is a collection of cells on the border of the atria and vena cava called the sinoatrial node. Heart cells exhibit automaticity which is the ability to generate electrical activity independent of external stimulation. As a result, the cells of the node spontaneously generate electrical activity that is subsequently conducted throughout the heart, resulting in a regular heart rate.[1]
In absence of any external stimuli, sinoatrial pacing contributes to maintain the heart rate in the range of 60-100 beats per minute (bpm).[8] At the same time, the two branches of the autonomic nervous system act in a complementary way increasing or slowing the heart rate. In this context, the vagus nerve acts on sinoatrial node slowing its conduction thus actively modulating vagal tone accordingly. This modulation is mediated by the neurotransmitter acetylcholine and downstream changes to ionic currents and calcium of heart cells.[4]
Given the evidence that the vagus nerve plays a crucial role in heart rate regulation by modulating the response of sinoatrial node, vagal tone can be quantified by investigating heart rate modulation induced by vagal tone changes. This kind of analysis allows to investigate vagal tone by means of several noninvasive techniques based on heart rate variability.[5]
Respiratory sinus arrhythmia
Respiratory sinus arrhythmia (RSA) is typically a benign, naturally occurring variation in heart rate that occurs during each breathing cycle. Specifically, heart rate increases during inspiration and decreases during expiration period.[1] RSA was firstly recognized by Carl Ludwig but its genesis and understanding it is still nowadays largely discussed.[9] RSA has been observed in humans from the early stages of life through adulthood.[10][1] Moreover, RSA is a mechanism which can be consistently found in several different species.[11][12][13]
During inhalation intra-thoracic pressure lowers due to the contraction and downward movement of the diaphragm and the expansion of the chest cavity. Atrial pressure is also lowered as a result, enabling an increased blood flow to the heart. Such increase in blood volume towards the heart cavities triggers baroreceptors which act to diminish vagal tone. Subsequently, heart rate increases.[1]
On the opposite during exhalation, the diaphragm relaxes, moving upward it decreases the size of the chest cavity, causing a subsequent increase in intrathoracic pressure. This increase in pressure inhibits venous return to the heart resulting in both reduced atrial expansion and minor activation of baroreceptors. Given the reduced baroreceptor activation, vagal tone is not suppressed as during inhalation so that it can exert its ability in decreasing heart rate.[1]
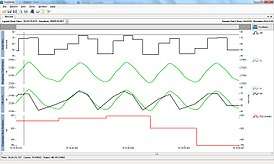
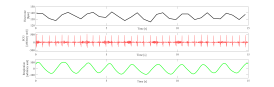
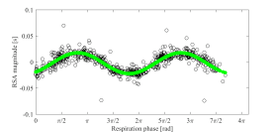
RSA as a vagal tone estimator
As previously described, it is nowadays established that the two divisions of the autonomic nervous system influence each other reciprocally and independently so more and more measures able to discriminate the two contributions have been developed. In recent years, several studies have been published highlighting the quantification of RSA as a reliable tool to investigate vagal tone in a noninvasive way. Such investigations encompass physiological, behavioral, and several clinical studies.[15][16][17] The main advantage in measuring of vagal tone by RSA is that such information are easily derivable from a single electrocardiography (ECG) recording.[18] At the same time, novel methodologies started addressing RSA quantification by a multivariate approach thus not considering ECG only but the interrelationship of ECG and respiration.[19][14]
On the opposite, vagal tone quantification by means of RSA has been questioned by many authors.[9][20] It has been argued that RSA is unequivocally related to vagal control but it also clear that is determined by two different mechanisms namely: vagal tonic and vagal phasic. The former processes exhibit different dynamics and origins so that it is crucial to be able to differentiate their contributions to RSA. Furthermore, it has been observed that tonic and phasic components are distinct yet not completely independent one each other.[1]
Despite the nowadays limitations in RSA quantification, it is considered a promising, noninvasive and reliable index of vagal control of the heart, thus an indirect estimator of vagal tone.[1]
Biological findings
The main hypothesis capable of explaining the reason behind the correlation of RSA and vagal tone describes RSA as an intrinsic resting function of the cardiopulmonary system.[21] The theory suggest that in animals and humans RSA may eventually contribute to energy saving for both cardiac and respiratory systems thus reducing the heart rate and related heartbeats numbers. Furthermore, RSA could save energy expenditure by suppressing ineffective ventilation during the ebb of perfusion (delivery of blood from arteries to capillaries for oxygenation and nutrition).[22][23]
In the physiological fields, RSA has been found to increase in subjects in resting state and to decrease in state of stress or tension.[21] RSA is increased in supine position and decreased in prone position. RSA is on average higher and more pronounced during day time with respect to night time.[21] RSA have also been extensively used to quantify vagal tone withdrawal in graded orthostatic tilt.[7][24]
Typically, expression of RSA decreases with age: it is pronounced in children and its magnitude tends to gradually disappear once a subject approach adulthood.[25] However, adults in excellent cardiovascular health, such as endurance runners, swimmers, and cyclists, are likely to have a more pronounced RSA. Professional athletes on average maintain very high vagal tone and consequently higher RSA levels. RSA has been found to becomes less prominent in individuals with diabetes and cardiovascular disease.[26]
Psychological findings
The majority of vagal tone research in the physiological field (social behavior, social interactions, and human psychology) have been focused on newborns and children.[25] The rational is to investigate children's adaptive functioning within a quantitative and reliable framework. Typically, researchers focus their attention on baseline vagal tone detection, treating it either as a potential predictor of behavior or examining its relationship with mental health (particularly emotion regulation, anxiety, and internalizing and externalizing disorders).[27]
The Polyvagal theory by Porges is considered as the most influential model able to describe the differences between basal vagal tone during steady state and vagal reactivity as a response to external stimuli.[28][29][30] The model describes vagal tone modifications a differential measure between vagal tone baseline and vagal tone activation during attention-demanding state. The theory states that successful vagal regulation is characterized by RSA suppression or withdrawal during attention tasks leading to increased metabolic output associated with heart rate increase.[25]
Despite the hypothesized link between vagal tone reduction and social functioning as stated by Porges' theory, researchers have been focusing mainly on the analysis of basal vagal tone. Examples are the findings reporting lower baseline RSA in children with Autism Spectrum Disorders with respect to healthy controls.[31] Research indicates that children with more secure attachments with their mothers exhibited greater empathetic responsiveness, less social inhibition, and higher vagal tone, highlighting the vagus nerve's regulatory effect, as well as the quantification of vagal tone by means of RSA, as a predictor of emotional and social function.[32]
Additional heart rate variability parameters
Vagal tone estimation based on heart rate is quantifiable by several parameters rather than the use of RSA only. Examples are indexes of beat-to-beat variability such as RMSSD reported by The Task Force of the European Society of Cardiology and Heart Rhythm Society.[33] Frequency analysis of heart rate in the range 0.15-0.4 Hz has been reported to quantify vagal tone based on heart rate variability spectrum.[24] In the specific context of vagal tone response to head up tilt, a measure of beat-to-beat variability (RMSSD) showed significant decreases following head-up tilts as reported by Myers.[34] Another method employed to quantify vagal activity is the computation of high frequency spectral component of heart rate variability power spectral density.[7][33] An example for the latter described methodology is the change in sympatho-vagal balance during hypnosis. Results report hypnosis to affect heart rate variability, shifting the sympatho-vagal interaction toward an enhanced parasympathetic activity and reduction of the sympathetic tone.[35]
See also
References
- 1 2 3 4 5 6 7 8 Berntson GG, Cacioppo JT, Quigley KS (March 1993). "Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications". Psychophysiology. 30 (2): 183–96. doi:10.1111/j.1469-8986.1993.tb01731.x. PMID 8434081.
- ↑ Diamond LM, Fagundes CP, Butterworth MR (2011). "Attachment Style, Vagal Tone, and Empathy During Mother-Adolescent Interactions". Journal of Research on Adolescence. 22 (1): 165–184. doi:10.1111/j.1532-7795.2011.00762.x.
- ↑ Grossman P, Wilhelm FH, Spoerle M (August 2004). "Respiratory sinus arrhythmia, cardiac vagal control, and daily activity". American Journal of Physiology. Heart and Circulatory Physiology. 287 (2): H728–34. doi:10.1152/ajpheart.00825.2003. PMID 14751862.
- 1 2 Howland RH (June 2014). "Vagus Nerve Stimulation". Current Behavioral Neuroscience Reports. 1 (2): 64–73. doi:10.1007/s40473-014-0010-5. PMC 4017164. PMID 24834378.
- 1 2 Porges SW, Doussard-Roosevelt JA, Maiti AK (2008). "Vagal tone and the physiological regulation of emotion". Monographs of the Society for Research in Child Development. 59 (2–3): 167–86. doi:10.1111/j.1540-5834.1994.tb01283.x. PMID 7984159.
- ↑ Brock C, Jessen N, Brock B, Jakobsen PE, Hansen TK, Rantanen JM, Riahi S, Dimitrova YK, Dons-Jensen A, Aziz Q, Drewes AM, Farmer AD (October 2017). "Cardiac vagal tone, a non-invasive measure of parasympathetic tone, is a clinically relevant tool in Type 1 diabetes mellitus". Diabetic Medicine. 34 (10): 1428–1434. doi:10.1111/dme.13421. PMID 28703868.
- 1 2 3 Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A (October 1994). "Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt". Circulation. 90 (4): 1826–31. doi:10.1161/01.CIR.90.4.1826. PMID 7923668.
- ↑ Nunan D, Sandercock GR, Brodie DA (November 2010). "A quantitative systematic review of normal values for short-term heart rate variability in healthy adults". Pacing and Clinical Electrophysiology. 33 (11): 1407–17. doi:10.1111/j.1540-8159.2010.02841.x. PMID 20663071.
- 1 2 De Burgh Daly M (1985), "Interactions Between Respiration and Circulation", Comprehensive Physiology, John Wiley & Sons, Inc., pp. 529–594, doi:10.1002/cphy.cp030216, ISBN 9780470650714
- ↑ Hathorn MK (April 1987). "Respiratory sinus arrhythmia in new-born infants". The Journal of Physiology. 385: 1–12. PMC 1192333. PMID 3656159.
- ↑ Myers MM, Fifer W, Haiken J, Stark RI (June 1990). "Relationships between breathing activity and heart rate in fetal baboons". The American Journal of Physiology. 258 (6 Pt 2): R1479–85. doi:10.1152/ajpregu.1990.258.6.R1479. PMID 2360694.
- ↑ Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T (August 1996). "Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency". Circulation. 94 (4): 842–7. PMID 8772709.
- ↑ Castellini MA, Rea LD, Sanders JL, Castellini JM, Zenteno-Savin T (November 1994). "Developmental changes in cardiorespiratory patterns of sleep-associated apnea in northern elephant seals". The American Journal of Physiology. 267 (5 Pt 2): R1294–301. doi:10.1152/ajpregu.1994.267.5.R1294. PMID 7977857.
- 1 2 Bartsch RP, Schumann AY, Kantelhardt JW, Penzel T, Ivanov PC (June 2012). "Phase transitions in physiologic coupling". Proceedings of the National Academy of Sciences of the United States of America. 109 (26): 10181–6. doi:10.1073/pnas.1204568109. PMC 3387128. PMID 22691492.
- ↑ Hayano J, Sakakibara Y, Yamada M, Kamiya T, Fujinami T, Yokoyama K, Watanabe Y, Takata K (March 1990). "Diurnal variations in vagal and sympathetic cardiac control". The American Journal of Physiology. 258 (3 Pt 2): H642–6. doi:10.1152/ajpheart.1990.258.3.H642. PMID 2316678.
- ↑ Porges SW (1986), "Respiratory Sinus Arrhythmia: Physiological Basis, Quantitative Methods, and Clinical Implications", Cardiorespiratory and Cardiosomatic Psychophysiology, Springer US, pp. 101–115, doi:10.1007/978-1-4757-0360-3_7, ISBN 9781475703627
- ↑ Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E (August 1986). "Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog". Circulation Research. 59 (2): 178–93. doi:10.1161/01.RES.59.2.178. PMID 2874900.
- ↑ Grossman P, van Beek J, Wientjes C (November 1990). "A comparison of three quantification methods for estimation of respiratory sinus arrhythmia". Psychophysiology. 27 (6): 702–14. PMID 2100356.
- ↑ Dick TE, Hsieh YH, Dhingra RR, Baekey DM, Galán RF, Wehrwein E, Morris KF (2014). "Cardiorespiratory coupling: common rhythms in cardiac, sympathetic, and respiratory activities". Progress in Brain Research. Elsevier. 209: 191–205. doi:10.1016/b978-0-444-63274-6.00010-2. ISBN 9780444632746. PMC 4052709. PMID 24746049.
- ↑ Grossman P, Karemaker J, Wieling W (March 1991). "Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control". Psychophysiology. 28 (2): 201–16. PMID 1946886.
- 1 2 3 Hayano J, Yasuma F (April 2003). "Hypothesis: respiratory sinus arrhythmia is an intrinsic resting function of cardiopulmonary system". Cardiovascular Research. 58 (1): 1–9. doi:10.1016/S0008-6363(02)00851-9. PMID 12667941.
- ↑ Ben-Tal A, Shamailov SS, Paton JF (April 2012). "Evaluating the physiological significance of respiratory sinus arrhythmia: looking beyond ventilation-perfusion efficiency". The Journal of Physiology. 590 (8): 1989–2008. doi:10.1113/jphysiol.2011.222422. PMC 3573317. PMID 22289913.
- ↑ Hayano J, Yasuma F, Okada A, Mukai S, Fujinami T (August 1996). "Respiratory sinus arrhythmia. A phenomenon improving pulmonary gas exchange and circulatory efficiency". Circulation. 94 (4): 842–7. doi:10.1161/01.cir.94.4.842. PMID 8772709.
- 1 2 Lewis GF, Furman SA, McCool MF, Porges SW (February 2012). "Statistical strategies to quantify respiratory sinus arrhythmia: are commonly used metrics equivalent?". Biological Psychology. 89 (2): 349–64. doi:10.1016/j.biopsycho.2011.11.009. PMC 3269511. PMID 22138367.
- 1 2 3 Graziano P, Derefinko K (September 2013). "Cardiac vagal control and children's adaptive functioning: a meta-analysis". Biological Psychology. 94 (1): 22–37. doi:10.1016/j.biopsycho.2013.04.011. PMC 4074920. PMID 23648264.
- ↑ Masi CM, Hawkley LC, Rickett EM, Cacioppo JT (February 2007). "Respiratory sinus arrhythmia and diseases of aging: obesity, diabetes mellitus, and hypertension". Biological Psychology. 74 (2): 212–23. doi:10.1016/j.biopsycho.2006.07.006. PMC 1804292. PMID 17034928.
- ↑ Connell AM, Hughes-Scalise A, Klostermann S, Azem T (October 2011). "Maternal depression and the heart of parenting: respiratory sinus arrhythmia and affective dynamics during parent-adolescent interactions". Journal of Family Psychology. 25 (5): 653–62. doi:10.1037/a0025225. PMID 21875198.
- ↑ Porges SW (July 1995). "Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory". Psychophysiology. 32 (4): 301–18. doi:10.1111/j.1469-8986.1995.tb01213.x. PMID 7652107.
- ↑ Porges SW (August 2003). "The Polyvagal Theory: phylogenetic contributions to social behavior". Physiology & Behavior. 79 (3): 503–13. PMID 12954445.
- ↑ Porges SW (December 2003). "Social engagement and attachment: a phylogenetic perspective". Annals of the New York Academy of Sciences. 1008: 31–47. PMID 14998870.
- ↑ Patriquin MA, Scarpa A, Friedman BH, Porges SW (March 2013). "Respiratory sinus arrhythmia: a marker for positive social functioning and receptive language skills in children with autism spectrum disorders". Developmental Psychobiology. 55 (2): 101–12. doi:10.1002/dev.21002. PMID 22212893.
- ↑ Diamond LM, Fagundes CP, Butterworth MR (2012). "Attachment style, vagal tone, and empathy during mother–adolescent interactions". Journal of Research on Adolescence. 22 (1): 165–184. doi:10.1111/j.1532-7795.2011.00762.x.
- 1 2 "Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology". European Heart Journal. 17 (3): 354–81. March 1996. PMID 8737210.
- ↑ Myers MM, Elliott AJ, Odendaal HJ, Burd L, Angal J, Groenewald C, Nugent JD, Yang JS, Isler JR, Dukes KA, Robinson F, Fifer WP (August 2017). "Cardiorespiratory physiology in the safe passage study: protocol, methods and normative values in unexposed infants". Acta Paediatrica. 106 (8): 1260–1272. doi:10.1111/apa.13873. PMC 5530586. PMID 28419567.
- ↑ DeBenedittis, G.; Cigada, M.; Bianchi, A.; Signorini, M. G.; Cerutti, S. (April 1994). "Autonomic changes during hypnosis: a heart rate variability power spectrum analysis as a marker of sympatho-vagal balance". The International Journal of Clinical and Experimental Hypnosis. 42 (2): 140–152. doi:10.1080/00207149408409347. ISSN 0020-7144. PMID 8200716.