Reactive nitrogen

Reactive nitrogen ("Nr") is a term used for a variety of nitrogen compounds that support growth directly or indirectly. Representative species include the gases nitrogen oxides (NOx), ammonia (NH3), nitrous oxide (N2O), as well as the anion nitrate (NO3−). Most of these species are the result of intensive farming, especially the (mis)use of fertilizers. Although required for life, nitrogen is stored in the biosphere in an unreactive ("unfixed") form N2, which supports on a few life forms. Reactive nitrogen is however "fixed" and is readily converted into protein, which supports life, leading to depletion of oxygen in fresh waters by eutrophication.[1]
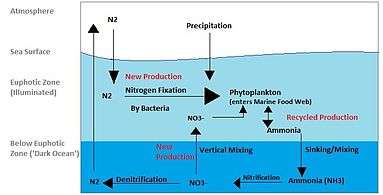
Reactive nitrogen compounds
In the environmental context, reactive nitrogen compounds include the following classes:
- oxide gases: nitric oxide, nitrogen dioxide, nitrous oxide. Containing oxidized nitrogen, mainly the result of industrial processes and internal combustion engines.
- anions: nitrate, nitrite. Nitrate is a common component of fertilizers, e.g. ammonium nitrate.
- amine derivatives: ammonia and ammonium salts, urea. Containing reduced nitrogen, these compounds are components of fertilizers.
All of these compounds enter into the nitrogen cycle.
As a consequence, an excess of Nr can affect the environment relatively quickly. This also means that nitrogen-related problems need to be looked at in an integrated manner.[2]
References
- Citations
- ↑ Sutton, Mark A.; Bleeker, Albert (2013). "Environmental science: The shape of nitrogen to come". Nature. 494 (7438): 435–437. Bibcode:2013Natur.494..435S. doi:10.1038/nature11954. PMID 23426258.
- ↑ http://international.vrom.nl/pagina.html?id=37594
- General references
- Hatfield, Jerry L; Follett, Ronald F (2008-07-16). Nitrogen in the environment: sources, problems, and management. Elsevier. ISBN 978-0-12-374347-3.
- Braun, Elizabeth; Division Of Technology, United Nations Environment Programme; Industry,; Economics,; Hole, Woods Hole Research Center (Woods; ), Mass; Initiative, International Nitrogen (2007). Reactive nitrogen in the environment: too much or too little of a good thing. UNEP/Earthprint. ISBN 978-92-807-2783-8.