Quassinoid
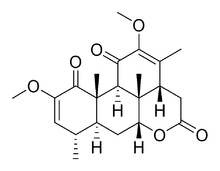
Chemical structure of quassin
Quassinoids are degraded triterpene lactones (similar to limonoids) of the Simaroubaceae plant family grouped into C-18, C-19, C-20, C-22 and C-25 types.[1] The prototypical member of the group, quassin, was first described in the 19th century from plants of the genus Quassia from which it gets its name.[2] It was isolated in 1937[3] and its structure elucidated in 1961.[4]
They are a biologically potent class of natural products, possessing antimalarial,[5] antifeedant,[6] insecticidal,[7] anti-inflammatory,[8] and anticancer[9] properties. The quassinoid bruceantin reached two separate phase II clinical trials in 1982[10] and 1983[11] but was deemed too cytotoxic to continue.
Other quassinoids include:[12]
- Bruceanols
- Bruceolide
- Eurycomanone
- Gutolactone
- Isobrucein A
- Neoquassin
- Nigakihemiacetal A
- Quassimarin
- Samaderines
- Simalikalactones
References
- ↑ Vieira, Studies in Natural Products Chemistry 2006 https://www.researchgate.net/profile/Ivo_Vieira/publication/251467805
- ↑ Winckler, F. L. (1835). Rep. Pharm. 4: 85. Missing or empty
|title=(help) - ↑ Clark, E. P. (1937). "Diarsyls. IX. Tetra-(3-amino-4-hydroxyphenyl)-diarsyl". J. Am. Chem. Soc. 59: 927. doi:10.1021/ja01284a045.
- ↑ Clark, E. P. (1937). "Diarsyls. IX. Tetra-(3-amino-4-hydroxyphenyl)-diarsyl". J. Am. Chem. Soc. 59: 927. doi:10.1021/ja01284a045.
- ↑ Muhammad, I.; Samoylenko, V. (2007). Expert Opin. Drug Discov. 2: 1065. Missing or empty
|title=(help) - ↑ Leskinen, V.; Polonsky, J.; Bhatnagar, S. (1984). J. Chem. Ecol. 10: 1497. Missing or empty
|title=(help) - ↑ Fang, X.; Di, Y. T.; Xhang, Y.; Xu, Z. P.; Lu, Y.; Chen, Q. Q.; Zheng, Q. T.; Hao, X. J. (2015). "Unprecedented Quassinoids with Promising Biological Activity fromHarrisonia perforata". Angew. Chem. Int. Ed. 54: 5992. doi:10.1002/anie.201412126.
- ↑ Hall, I. H.; Lee, K. H.; Imakura, Y.; Okano, M.; Johnson, A. (1983). J. Pharm. Sci. 72: 1282. Missing or empty
|title=(help) - ↑ Fukamiya, N.; Lee, K.H.; Muhammad, I., Murakami, C.; Okano, M.; Harvey, I.; Pelletier, J. (2005). "Structure–activity relationships of quassinoids for eukaryotic protein synthesis". Cancer Letters. 220: 37. doi:10.1016/j.canlet.2004.04.023.
- ↑ Wiseman, C. L.; Yap, H. Y.; Bedikian, A. Y.; Bodey, G. P.; Blumenchein, G. R. (1982). Am. J. Clin. Oncol. 5: 389. Missing or empty
|title=(help) - ↑ Arsenau, J. C.; Wolter, J. M.; Kuperminc, M.; Ruckdeschel, J. C. (1983). Invest. New Drugs. 1: 239. Missing or empty
|title=(help) - ↑ "Quassinoid". Chemical Entities of Biological Interest (ChEBI).
External links


This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.