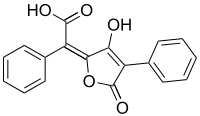
Pulvinic acid
 | |
| Names | |
|---|---|
| IUPAC name
(2E)-(5-Hydroxy-3-oxo-4-phenyl-2(3H)-furanylidene)(phenyl)acetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C18H12O5 | |
| Molar mass | 308.29 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pulvinic acid is a natural chemical pigment found in some lichens.[1] Dimers of pulvinic acid have been found in the fungi Scleroderma citrinum and Chalciporus piperatus.[2] Hydroxypulvinic acid pigments (pulvinic acid type family of pigments) have been found in Boletus (e.g. Boletus erythropus), Boletinus, Chalciporus, Gyrodon, Leccinum, Pulveroboletus, Suillus (e.g. Suillus luteus, Suillus bovinus, and Suillus grevillei), Paxillus (e.g. Paxillus involutus), Serpula (e.g. Serpula lacrymans), Xerocomus (e.g. Xerocomus chrysenteron), Hygrophoropsis (e.g. Hygrophoropsis aurantiaca), Retiboletus (e.g. Retiboletus nigerrimus), Pulveroboletus (e.g. Pulveroboletus auriflammeus), and are generally characteristic of Boletales.[3][4] Derivatives and similar pigments of this family include atromentic acid, xerocomic acid, isoxerocomic acid, variegatic acid, variegatorubin, xerocomorubin, chinomethide, methyl bovinate, pulvinone, badion A, norbadion A, bisnorbadiochinone A, pisochinone, and sclerocitrin.[3][4]
See also
References
- ↑ Bourdreux, Yann; Bodio, Ewen; Willis, Catherine; Billaud, Célia; Le Gall, Thierry; Mioskowski, Charles (2008). "Synthesis of vulpinic and pulvinic acids from tetronic acid". Tetrahedron. 64 (37): 8930–8937. doi:10.1016/j.tet.2008.06.058.
- ↑ Winner M, Giménez A, Schmidt H, Sontag B, Steffan B, Steglich W (2004). "Unusual pulvinic acid dimers from the common fungi Scleroderma citrinum (common earthball) and Chalciporus piperatus (peppery bolete)". Angewandte Chemie. 43 (14): 1883–6. doi:10.1002/anie.200352529. PMID 15054803.
- 1 2 https://edoc.ub.uni-muenchen.de/783/1/Gruber_Gertraud.pdf
- 1 2 Gill, M., and Steglich, W. (1987) Pigments of fungi (Macromycetes). Prog Chem Org Nat Prod 51: 1–317.