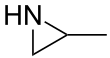
Propyleneimine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Methylaziridine | |||
| Other names
1,2-Propylenimine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.799 | ||
| EC Number | 613-033-00-6 | ||
PubChem CID |
|||
| RTECS number | CM8050000 | ||
| UN number | 1921 (inhibited) | ||
| |||
| |||
| Properties | |||
| C3H7N | |||
| Molar mass | 57.10 g·mol−1 | ||
| Appearance | Colorless, oily liquid[1] | ||
| Odor | ammonia-like[1] | ||
| Density | 0.9 g/mL[2] | ||
| Melting point | −63 °C (−81 °F; 210 K)[2] | ||
| Boiling point | 67 °C (153 °F; 340 K)[2] | ||
| Miscible[2] | |||
| Vapor pressure | 112 mmHg (20°C)[1] | ||
| Hazards | |||
| R-phrases (outdated) | R45-R11-R26/27/28-R41[2] | ||
| S-phrases (outdated) | S53-S45[2] | ||
| Flash point | −4 °C (25 °F; 269 K)[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published) |
500 ppm (rat, 4 hr)[3] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
TWA 2 ppm (5 mg/m3) [skin][1] | ||
REL (Recommended) |
Ca TWA 2 ppm (5 mg/m3) [skin][1] | ||
IDLH (Immediate danger) |
Ca [100 ppm][1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Propyleneimine is the organic compound with the formula CH3CH(NH)CH2. It is a secondary amine]] and the smallest chiral aziridine (ring containing C2N). It is a colorless liquid.
The compound is mainly of academic interest, e.g. for the synthesis of dendrimers, a process that exploits the tendency of aziridines to undergo ring-opening reactions.[4] [2]
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0537". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 5 6 7 8 Propyleneimine International Chemical Safety Card at actrav.itcilo.org
- ↑ "Propylene imine". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J. W.; Meijer, E. W.; Paulus, W.; Duncan, R. (2000). "Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labeled polyamidoamine dendrimers in vivo". Journal of Controlled Release. 65: 133–148. doi:10.1016/S0168-3659(99)00246-1.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.