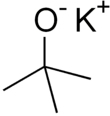
Potassium ''tert''-butoxide
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
potassium 2-methylpropan-2-olate | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.011.583 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C4H9KO | |||
| Molar mass | 112.21 g mol−1 | ||
| Appearance | solid | ||
| Melting point | 256 °C (493 °F; 529 K) | ||
| Solubility in diethyl ether | 4.34 g/100 g (25-26 °C) [1] | ||
| Solubility in Hexane | 0.27 g/100 g (25-26 °C) [1] | ||
| Solubility in Toluene | 2.27 g/100 g (25-26 °C) [1] | ||
| Solubility in THF | 25.00 g/100 g (25-26 °C) [1] | ||
| Hazards | |||
| Safety data sheet | Oxford MSDS | ||
EU classification (DSD) (outdated) |
Harmful (Xn), Corrosive (C) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
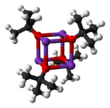
Potassium tert-butoxide is the chemical compound with the formula K+(CH3)3CO−. This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. It exists as a tetrameric cubane-type cluster. It is often seen written in chemical literature as potassium t-butoxide. The compound is often depicted as a salt, and it often behaves as such, but it is not ionized in solution.
Preparation
Potassium t-butoxide is commercially available as a solution and as a solid, but it is often generated in situ for laboratory use because samples are so sensitive and older samples are often of poor quality. It is prepared by the reaction of dry tert-butyl alcohol with potassium metal.[2] The solid is obtained by evaporating these solutions followed by heating the solid. The solid can be purified by sublimation at 220 °C and 1 mmHg.
Structure
Potassium tert-butoxide crystallises from THF/pentane at −20 °C as [tBuOK·tBuOH]∞, which consists of infinite one-dimensional chains linked by hydrogen bonding. Sublimation of [tBuOK·tBuOH]∞ affords the tetramer [tBuOK]4, which adopts a cubane-like structure. Mild Lewis basic solvents such as THF and diethyl ether do not break up the tetrameric structure, which persists in the solid, in solution and even in the gas phase.[3]
Applications
The tert-butoxide species is itself useful as a strong, non-nucleophilic base in organic chemistry.[4] It is not as strong as amide bases, e.g. lithium diisopropylamide, but stronger than potassium hydroxide. Its steric bulk inhibits the group from participating in nucleophilic addition, such as in a Williamson ether synthesis or an SN2 reaction. Substrates that are deprotonated by potassium t-butoxide include terminal acetylenes and active methylene compounds. It is useful in dehydrohalogenation reactions.
Potassium tert-butoxide catalyzes the reaction of hydrosilanes and heterocyclic compounds to give the silyl derivatives, with release of H2.[5]
Modifications
Many modifications have been reported that influence the reactivity of this reagent. The compound adopts a complex cluster structure (the adjacent picture is a simplified cartoon), and additives that modify the cluster affect the reactivity of the reagent. For example, DMF, DMSO, hexamethylphosphoramide (HMPA), and 18-crown-6 interact with the potassium center, enhancing the basicity of the butoxide. Schlosser's base, a mixture of the alkoxide and an alkyl lithium compound, is a related but stronger base.[4]
Reactions
Potassium tert-butoxide reacts with chloroform yielding dichlorocarbene,[6] the reaction can result in ignition.[7] Potassium tert-butoxide should never be added to dichloromethane.[8]
References
- 1 2 3 4 Caine D. (2006). "Potassium tert-Butoxide". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp198.pub2.
- ↑ William S. Johnson and William P. Schneider (1963). "β-Carbethoxy-γ,γ-diphenylvinylacetic acid". Organic Syntheses. ; Collective Volume, 4, p. 132
- ↑ Chisholm, Malcolm H.; Drake, Simon R.; Naiini, Ahmad A.; Streib, William E. (1991). "Synthesis and X-ray crystal structures of the one-dimensional ribbon chains [MOBut·ButOH]∞ and the cubane species [MOBut]4 (M = K and Rb)". Polyhedron. 10 (3): 337–345. doi:10.1016/S0277-5387(00)80154-0.
- 1 2 Drury Caine "Potassium t-Butoxide" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, New York, 2006. doi: 10.1002/047084289X.rp198.pub2. Article Online Posting Date: September 15, 2006
- ↑ Anton A. Toutov, Wen-Bo Liu, Kerry N. Betz, Alexey Fedorov, Brian M. Stoltz, Robert H. Grubbs (2015). "Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst". Nature. 518: 80–84. doi:10.1038/nature14126.
- ↑ Brown, William; Foote, Christopher; Iverson, Brent; Anslyn, Eric (2008-01-10). Organic Chemistry. Cengage Learning. ISBN 0495388572.
- ↑ Armour, Margaret-Ann (2016-04-19). Hazardous Laboratory Chemicals Disposal Guide, Third Edition. CRC Press. ISBN 9781420032383.
- ↑ Foden, Charles R.; Weddell, Jack L. (1991-12-29). Hazardous Materials: Emergency Action Data. CRC Press. ISBN 9780873715980.