Potassium dicyanoaurate
-3D-balls.png) Ball-and-stick model of the aurocyanide or dicyanoaurate(I) complex anion, [Au(CN)2]−.[1] | |
| Names | |
|---|---|
| Other names
potassium dicyanoaurate(I) | |
| Identifiers | |
3D model (JSmol) |
|
| 6235525 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.303 |
| EC Number | 237-748-4 |
| 37363 | |
PubChem CID |
|
| UNII | |
| UN number | 1588 |
| |
| |
| Properties | |
| C2AuKN2 | |
| Molar mass | 288.10 g·mol−1 |
| Appearance | white powder |
| Boiling point | decomposes |
| water-soluble | |
| Hazards | |
| Main hazards | toxic |
| GHS pictograms | 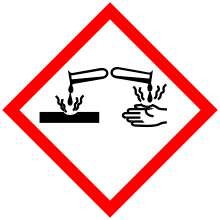   |
| GHS signal word | Warning |
| H300, H400, H310, H410, H330, H317, H290, H318, H315 | |
| P260, P264, P273, P280, P284, P301+310 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Potassium dicyanoaurate, also potassium dicyanoaurate(I), potassium gold cyanide, potassium gold dicyanide or gold potassium cyanide, is an inorganic compound with formula K[Au(CN)2]. It is a colorless to white crystalline powder, usually prepared by dissolving metallic gold in aqueous solution of potassium cyanide. It is most often used in gold plating applications. It contains 68.2 wt.% of gold. It is soluble in water and slightly soluble in alcohol.
Its CAS number is 13967-50-5 and its EC number number is 237-748-4. Its molar mass is 288.33 g/mol, its density is 3.45 g/cm3.
Potassium gold cyanide can be used for photoreduction of gold ions by nanopowder ZnO, preparation of gold-gold junction electrodes in voltammetric glucose detection,[2] and other reactions where metallic gold is prepared.
A trivalent compound, potassium tetracyanoaurate(III), K[Au(CN)4], also exists, but its use is less common.
The cyanide-gold complex penetrates cells much easier than gold ions alone, facilitating gold toxicity. Gold inhibits activity of many enzymes, hindering detoxification of the cyanide ion to thiocyanate, potentiating the cyanide toxicity.[3]
Gold mining
Potassium dicyanoaurate is produced in the cyanide process variant of gold mining.

The chemical reaction for the dissolution of gold, the "Elsner Equation", follows:
4 Au + 8 KCN + O2 + 2H2O → 4 K[Au(CN)2] + 4 KOH
In this redox process, oxygen removes, via a two-step reaction, one electron from each gold atom to form the complex Au(CN)−
2 ion.[4]
References
- ↑ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ https://www.alfa.com/en/catalog/012552/
- ↑ Wright, I. H.; Vesey, C. J. (September 1986). "Acute poisoning with gold cyanide". Anaesthesia. 41 (9): 936–939. doi:10.1111/j.1365-2044.1986.tb12920.x.
- ↑ (Web Archive) Technical Bulletin, https://web.archive.org/web/20091023235047/http://www.multimix.com.au/DOCUMENTS/Technical%20Bulletin1.PDF