Polythiazyl
 | |
x.png) | |
 | |
| Names | |
|---|---|
| Other names
polythiazyl poly(sulfur nitride) | |
| Identifiers | |
| ChemSpider |
|
| Properties | |
| (SN)x | |
| Appearance | bronze colour, metallic lustre[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
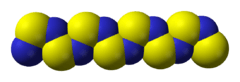
Polythiazyl (polymeric sulfur nitride), (SN)x, is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered[1] and was also found to be a superconductor at very low temperatures (below 0.26 K).[2][3] It is a fibrous solid, described as "lustrous golden on the faces and dark blue-black", depending on the orientation of the sample. It is air stable and insoluble in all solvents.[4]
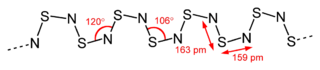
Structure and bonding
The material is a polymer. The S and N atoms on adjacent chains align.[5] Several resonance structures can be written.[6]
Synthesis
Polythiazyl is synthesized by the polymerization of the dimer disulfur dinitride (S2N2), which is in turn synthesized from the cyclic alternating tetramer tetrasulfur tetranitride (S4N4). Conversion from cyclic tetramer to dimer is catalysed with hot silver wool.[1][7]
- S4N4 + 8 Ag → 4 Ag2S + 2 N2
- S4N4 (w/ Ag2S catalyst) → 2 S2N2 (w/ 77K cold finger) → S2N2
- S2N2 (@ 0°C, sublimes to surface) → thermal polymerization → (SN)x
Uses
Due to its electrical conductivity, polythiazyl is used in LEDs, transistors, battery cathodes, and solar cells.[7]
References
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 725–727. ISBN 0-08-037941-9.
- ↑ Labes, M. M.; Love, P.; Nichols, L. F. (1979). "Polysulfur Nitride - a Metallic, Superconducting Polymer". Chemical Reviews. 79 (1): 1–15. doi:10.1021/cr60317a002.
- ↑ Harry R. Allcock (20 September 2011). Introduction to Materials Chemistry. John Wiley & Sons. p. 131. ISBN 978-1-118-21098-7. Retrieved 29 June 2012.
- ↑ A. G. MacDiarmid; C. M. Mikulsk; A. J. Heeger; A. F. Garito (1983). "Polymeric Sulfur Nitride (Polythiazyl), (SN)x". Inorganic Syntheses. 22: 143. doi:10.1002/9780470132531.ch31.
- ↑ Cohen, M.J .; Garito, A. F.; Heeger, A. J.; MacDiarmid, A. G.; Mikulski, C. M.; Saran, M. S.; Kleppinger, J. (1976). "Solid state polymerization of S2N2 to (SN)x". Journal of the American Chemical Society. 98: 3844–3848. doi:10.1021/ja00429a018.
- ↑ Okada, M.; Tanaka, K.; Takata, A.; Yamabe, T. (1993). "Examination of Electronic Phase of the Hartree-Fock Solution of an Isolated Polythiazyl Chain". Synthetic Metals. 59 (2): 223–230. doi:10.1016/0379-6779(93)91029-2.
- 1 2 Ronald D. Archer (26 February 2001). Inorganic and Organometallic Polymers. John Wiley & Sons. p. 213. ISBN 978-0-471-24187-4. Retrieved 29 June 2012.
