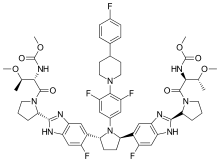
Pibrentasvir
 | |
| Clinical data | |
|---|---|
| Trade names | Mavyret, Maviret (combination with glecaprevir) |
| Synonyms | ABT-530 |
| Pharmacokinetic data | |
| Protein binding | >99.9% |
| Elimination half-life | 13 hours |
| Excretion | 96.6% in faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C57H65F5N10O8 |
| Molar mass | 1,113.20 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pibrentasvir is an antiviral agent.[1] In the United States and Europe, it is approved for use with glecaprevir as the combination drug glecaprevir/pibrentasvir (trade name Mavyret in the US and Maviret in the EU) for the treatment of hepatitis C.[2][3] It is sold by Abbvie.
References
- ↑ Ng, Teresa I.; Krishnan, Preethi; Pilot-Matias, Tami; Kati, Warren; Schnell, Gretja; Beyer, Jill; Reisch, Thomas; Lu, Liangjun; Dekhtyar, Tatyana; Irvin, Michelle; Tripathi, Rakesh; Maring, Clarence; Randolph, John T.; Wagner, Rolf; Collins, Christine (2017). "In Vitro Antiviral Activity and Resistance Profile of the Next-Generation Hepatitis C Virus NS5A Inhibitor Pibrentasvir". Antimicrobial Agents and Chemotherapy. 61 (5): e02558–16. doi:10.1128/AAC.02558-16. PMID 28193664.
- ↑ Linda A. Johnson (August 3, 2017). "FDA OKs new drug to treat all forms of hepatitis C". Fox Business.
- ↑ "Maviret: EPAR – Summary for the public" (PDF). European Medicines Agency. 2017-08-17.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.