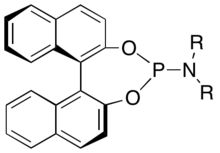
Phosphoramidite ligand
A phosphoramidite ligand is a chiral monodentate phosphine ligand, widely used for enantioselective synthesis. They were invented by Dutch chemist Ben Feringa. The introduction of phosphoramidite ligands challenged the notion that high flexibility in the metal–ligand complex is detrimental for high stereo control.
History
In 1996 Feringa et. al, reported an enantioselective 1,4-addition of aliphatic zinc reagents to enones catalyzed by copper in the presence of BINOL derived phosphoramidite ligands.[1][2] The high levels of enantioselectivity achieved with these ligands opened the door for numerous other asymmetric transformations, which previously relied on bidentate phosphine ligands. This paradigm shift using monodentate phosphine ligands challenged the belief that a tight metal-ligand complex is necessary for high stereocontrol. Then in 2000, Reetz,[3] Pringle, [4] and Feringa and de Vries [5] each autonomously described the use of phosphoramidite ligands for asymmetric hydrogenation. High enantioselectivities were reached, rivaling those using the most selective bidentate ligands known. Various chiral commodities could be obtained via this synthetic method including, amino acids, diacids and esters, cinnamic acids, amines, as well as heterocycles.
Synthesis of phosphoramidite ligands
Phosphoramidite ligands bearing the BINOL backbone (Figure 2.) are prepared via the chlorophosphite. [6] The two hydroxyl groups of BINOL are treated first with phosphorus trichloride and subsequently the desired amine (HNR2) is added in the presence of a base.
Reactions using phosphoramidite ligands
1,4-addition
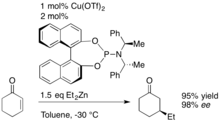
A dramatic increase in the enantioselectivity of the addition reactions was observed when a chiral amine moiety was incorporated in these phosphoramidite ligands. Excellent yields (up to 95 %) and ee values exceeding 98 % ee were achieved in the addition to cyclochexenone in the presence of the bis(1-phenylethyl)amine derived ligand. [7] In addition to the organozinc nucleophiles used by Feringa, the 1,4-addition to enones has been shown by Woodward[8] to work with organoaluminum nucleophiles.
Rhodium-Catalyzed Hydrogenation
Phosphoramidite ligands also find use in asymmetric hydrogenation reactions. The ligands for this transformation require generating a chiral center on the amine portion of the ligand. These ligands afford a very active and productive catalyst, which efficiently reduces a various acetamides.[9] It is worth noting that the ligand shown (Figure 4.) is the only ligand known to afford greater than 90% enantioslectivity for the substrate shown.
Asymmetric Regioselective Allylic Aminations
Hartwig and co-workers have succeeded in developing highly selective iridium catalysts with (R,R,R)-phosphoramidite L[10] The allylic aminations of a wide variety of achiral allylic esters proceeded with total conversion and superb regioselectivity in many cases. The reaction shown clearly illustrates the power of this methodology, wherein cinnamyl acetate was converted to the allylic benzyl amine in excellent yield and enantiopurity . The authors mentioned that these valuable amination reactions were mediated by air-stable Ir complexes at ambient temperatures, which should lead to wide acceptance of this catalyst in bench-top organic synthesis.
References
- ↑ A. H. M. de Vries, A. Meetsma, B. L. Feringa, Angew. Chem.1996, 108, 2526; Angew. Chem. Int. Ed. Engl. 1996, 35, 2374.
- ↑ B. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos, A. H. M.de Vries, Angew. Chem. 1997, 109, 2733
- ↑ M. T. Reetz, T. Sell, Tetrahedron Lett. 2000, 41, 6333.
- ↑ C. Claver, E. Fernandez, A. Gillon, K. Heslop, D. J. Hyett, A.Martorell, A. G. Orpen, P. G. Pringle, Chem. Commun. 2000,961.
- ↑ M. van den Berg, A. J. Minnaard, E. P. Schudde, J. van Esch,A. H. M. de Vries, J. G. de Vries, B. L. Feringa, J. Am. Chem.Soc. 2000, 122, 11539.
- ↑ B. L. Feringa, Acc. Chem. Res. 2000, 33, 346.
- ↑ A. H. M. de Vries, A. Meetsma, B. L. Feringa, Angew. Chem.1996, 108, 2526; Angew. Chem. Int. Ed. Engl. 1996, 35, 2374.
- ↑ Alexakis, A.; Albrow, V.; Biswas, K.; Augustin, M.; Prieto, O.; Woodward, S. Chem. Commun., 2005, 2843.
- ↑ Pena, D. et al. J. Am. Chem. Soc. 2002, 124, 14552.
- ↑ Hartwig, J. F. et al. J. Am. Chem. Soc. 2002, 124, 15164.