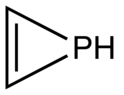
Phosphirene
 | |
| Names | |
|---|---|
| IUPAC name
1H-Phosphirene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C2H3P | |
| Molar mass | 58.02 g·mol−1 |
| Appearance | colorless gas |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphirene is the organophosphorus compound with the formula C2H2PH. It is a colorless gas of no commercial value. As the simplest cyclic, unsaturated organophosphorus compound, phosphirene is the prototype of a family of related compounds that have attracted attention from researchers.[1]
Phosphirenes, that is substituted phosphirene compounds where one or more of the H's are replaced organic substituents, are far more commonly discussed than the parent phosphirene. The first example of a phosphirene, 1,2,3-triphenylphosphirene was prepared via trapping of the phosphinidine complex Mo(CO)5PPh with diphenylacetylene.[2]
References
- ↑ François Mathey, Manfred Regitz (1996). "Phosphiranes, Phosphirenes, and Heavier Analogues". Comprehensive Heterocyclic Chemistry II. 1A. pp. 277–304. doi:10.1016/B978-008096518-5.00008-3.
- ↑ Angela Marinetti, Francois Mathey, Jean Fischer, Andre Mitschler (1982). "Generation and Trapping of Terminal Phosphinidene Complexes. Synthesis and X-ray Crystal Structure of Stable Phosphirene Complexes". J. Am. Chem. Soc. 104. pp. 4484–5. doi:10.1021/ja00380a029.
- Quin, L. D. (2000). A Guide to Organophosphorus Chemistry. Wiley-Interscience. ISBN 0-471-31824-8.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.