Phosphinous acid
 | |
| Names | |
|---|---|
| Other names
hydroxyphosphine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| H3OP | |
| Molar mass | 50.00 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
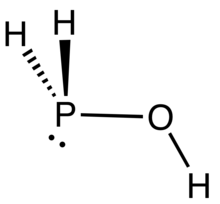
Phosphinous acid is the inorganic compound with the formula H2POH. It exists, fleetingly, as a mixture with its less stable tautomer H3PO. These species have been generated by low temperature oxidation of phosphine with ozone.[1] H2POH is mainly of pedagogical interest. Many phosphinous acids are known containing organic substituents. One example is diphenylphosphinous acid.
References
- ↑ Withnall, Robert; Andrews, Lester (1987). "FTIR spectra of the photolysis products of the phosphine-ozone complex in solid argon". Journal of Physical Chemistry. 91 (4): 784–97. doi:10.1021/j100288a008.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.