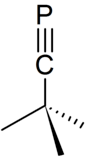
Phosphaalkyne
In chemistry, phosphaalkynes (IUPAC name: alkylidynephosphanes) are organophosphorus compounds that have a phosphorus-carbon triple bond, giving them a general formula of (R-C≡P).[1]
Structure and properties
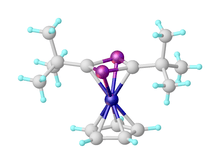
Phosphaalkynes are heavy structural analogues of nitriles (R-C≡N), i.e. P replaces N in a nitrile. Their reactions are more similar to those of alkynes than nitriles, for example undergoing 1,2-addition reactions and cycloaddition reactions.[1] The presence of the phosphorus lone pair allows then to act as ligands, with their organometallic chemistry being the subject of significant study.[3][4] Illustrative of the ease of the cyclization reactions, CpCo(C2H4)2 reacts below room temperature with t-BuCP to give good yields of CpCo(P2C2(t-Bu)2).[2]
History
In 1950, H. Albers reported the first indication of the existence of H-C≡P. This compound was identified by infrared absorption spectrometry, and its synthesis was improved by Manfred Regitz in 1987. The synthesis of the first kinetically stable phosphaalkyne, tert-Butylphosphaacetylene), was reported in 1981 by Gerd Becker and Werner Uhl.
See Also
- Cyaphide - The corresponding P≡C− anion.
References
| Wikimedia Commons has media related to Phosphaalkynes. |
- 1 2 Regitz, M. (1990). "Phosphaalkynes: New Building Blocks in Synthetic Chemistry". Chemical Reviews. 90: 191–213. doi:10.1021/cr00099a007.
- 1 2 Binger, P.; Milczarek, R.; Mynott, R.; Krüger, C.; Tsay, Y.-H.; Raabe, E.; Regitz, M. (1988). "λ3-1,3-Diphosphacyclobutadiene-Cobalt Complexes by Cyclodimerization of λ3-Phosphaalkynes". Chemische Berichte. 121: 637–645. doi:10.1002/cber.19881210409.
- ↑ Nixon, J. F. (1995). "Recent Developments in the Organometallic Chemistry of Phospha-Alkynes, RC≡P". Coordination Chemistry Reviews. 145: 201–258. doi:10.1016/0010-8545(95)90224-4.
- ↑ Nixon, John F. (1995). "Phospha-alkynes, RC≡P: new building blocks in inorganic and organometallic chemistry". Chem. Soc. Rev. 24 (5): 319–328. doi:10.1039/CS9952400319.