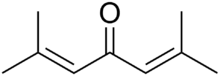
Phorone
 | |
| Names | |
|---|---|
| IUPAC name
2,6-Dimethyl-2,5-heptadien-4-one | |
| Other names
Phorone Diisopropylidene acetone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.261 |
PubChem CID |
|
| |
| |
| Properties | |
| C9H14O | |
| Molar mass | 138.20 g/mol |
| Density | 0.885 g/cm3 |
| Melting point | 28 °C (82 °F; 301 K) |
| Boiling point | 198 to 199 °C (388 to 390 °F; 471 to 472 K) |
| Hazards | |
| Safety data sheet | External MSDS |
| Flash point | 79 °C (174 °F; 352 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phorone, or diisopropylidene acetone, is a yellow crystalline substance with a geranium odor. It is a self-condensation product of acetone and a common impurity in mesityl oxide.[1]
Preparation
It was first obtained in 1837 in impure form by the French chemist Auguste Laurent, who called it "camphoryle".[2] In 1849, the French chemist Charles Frédéric Gerhardt and his student Jean Pierre Liès-Bodart prepared it in a pure state and named it "phorone".[3] On both occasions it was produced by ketonization through the dry distillation of the calcium salt of camphoric acid.[4][5]
It is now typically obtained by the reaction of acetone with hydrogen chloride.
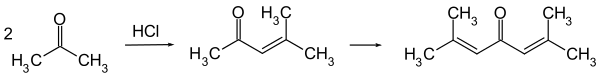
Crude phorone can be purified by repeated recrystallization from ethanol or ether, in which it is soluble.
Reactions
Phorone can condense with ammonia to form triacetone amine.
See also
References
- Merck Index, 11th Edition, 7307.
- ↑ Hardo Siegel, Manfred Eggersdorfer (2005). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.
- ↑ Laurent, Auguste (1837). "Sur les acides pinique et sylvique, et sur le camphoryle" [On pinic and sylvic acids, and on camphoryl]. Annales de Chimie et de Physique. 2nd series (in French). 65: 324–332. ; see "Camphoryle", pp. 329–330.
- ↑ See:
- Gerhardt, Charles (1849) Comptes rendus des travaux de chimie (Paris, France: Masson, 1849), p. 385. (in French)
- Gerhardt; Liès-Bodart (1849). "Trockne Destillation des camphorsauren Kalks" [Dry distillation of calcium camphorate]. Annalen der Chemie und Pharmacie (in German). 72: 293–294. doi:10.1002/jlac.18490720327. From p. 293: "Dieses Oel, welches Gerhardt und Lies-Bodart mit dem Namen Phoron bezeichnen, … " (This oil, which Gerhardt and Liès-Bodart designate by the name "phorone", … )
- ↑ Watts, Henry, A Dicitonary of Chemistry and the Allied Branches of Other Sciences (London, England: Longmans, Green, and Co., 1863), vol. 1, "Camphorone", p. 733.
- ↑ Kekulé, August (1866). Lehrbuch der organischen Chemie [Textbook of organic chemistry] (in German). 2nd vol. Erlangen, (Germany): Ferdinand Enke. p. 463.