Periodinane
Periodinanes also known as λ5-iodanes are organoiodine compounds with iodine in the +5 oxidation state.
These compounds are hypervalent because the iodine atoms in them formally contain more than 8 valence electrons.
Periodinane compounds
The λ5-iodanes such as the Dess-Martin periodinane have square pyramidal geometries with 4 heteroatoms in basal positions and one apical phenyl group.
Iodoxybenzene or iodylbenzene, C6H5IO2, is a known oxidizing agent. It was first prepared by Willgerodt by disproportionation of iodosylbenzene under steam distillation to iodylbenzene and iodobenzene:
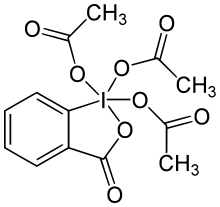
Dess-Martin periodinane (1983) is another powerful oxidant and an improvement of the IBX acid already in existence in 1983. The IBX acid is prepared from 2-iodobenzoic acid and potassium bromate and sulfuric acid [1] and is insoluble in most solvents whereas the Dess-Martin reagent prepared from reaction of the IBX acid with acetic anhydride is very soluble. The oxidation mechanism ordinarily consists of a ligand exchange reaction followed by a reductive elimination.
Uses
The predominant use of periodinanes is as oxidizing reagents replacing toxic reagents based on heavy metals.[2]
See also
References
- ↑ Robert K. Boeckman, Jr., Pengcheng Shao, and Joseph J. Mullins. "1,2-Benziodoxol-3(1H)-one, 1,1,1-tris(acetyloxy)-1,1-dihydro-". Organic Syntheses. ; Collective Volume, 10, p. 696
- ↑ Hypervalent iodine(V) reagents in organic synthesis Uladzimir Ladziata and Viktor V. Zhdankin Arkivoc 05-1784CR pp 26-58 2006 Article