PIK3C2A
Phosphatidylinositol-4-phosphate 3-kinase C2 domain-containing alpha polypeptide is an enzyme that in humans is encoded by the PIK3C2A gene.[5][6]
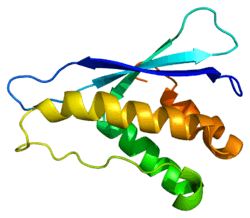
The protein encoded by this gene belongs to the phosphoinositide 3-kinase (PI3K) family. PI3-kinases play roles in signaling pathways involved in cell proliferation, oncogenic transformation, cell survival, cell migration, and intracellular protein trafficking. This protein contains a lipid kinase catalytic domain as well as a C-terminal C2 domain, a characteristic of Class II PI 3-kinases. C2 domains act as calcium-dependent phospholipid binding motifs that mediate translocation of proteins to membranes, and may also mediate protein-protein interactions. The PI3-kinase activity of this protein is not sensitive to nanomolar levels of the inhibitor wortmannin. This protein was shown to be able to be activated by insulin and may be involved in integrin-dependent signaling.[6]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000011405 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000030660 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Domin J, Pages F, Volinia S, Rittenhouse SE, Zvelebil MJ, Stein RC, Waterfield MD (Nov 1997). "Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin". Biochem J. 326 (1): 139–47. PMC 1218647. PMID 9337861.
- 1 2 "Entrez Gene: PIK3C2A phosphoinositide-3-kinase, class 2, alpha polypeptide".
Further reading
- Lee C, Liu QH, Tomkowicz B, et al. (2004). "Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways". J. Leukoc. Biol. 74 (5): 676–682. doi:10.1189/jlb.0503206. PMID 12960231.
- Milani D, Mazzoni M, Borgatti P, et al. (1996). "Extracellular human immunodeficiency virus type-1 Tat protein activates phosphatidylinositol 3-kinase in PC12 neuronal cells". J. Biol. Chem. 271 (38): 22961–22964. doi:10.1074/jbc.271.38.22961. PMID 8798481.
- Mazerolles F, Barbat C, Fischer A (1997). "Down-regulation of LFA-1-mediated T cell adhesion induced by the HIV envelope glycoprotein gp160 requires phosphatidylinositol-3-kinase activity". Eur. J. Immunol. 27 (9): 2457–2465. doi:10.1002/eji.1830270946. PMID 9341793.
- Borgatti P, Zauli G, Colamussi ML, et al. (1998). "Extracellular HIV-1 Tat protein activates phosphatidylinositol 3- and Akt/PKB kinases in CD4+ T lymphoblastoid Jurkat cells". Eur. J. Immunol. 27 (11): 2805–2811. doi:10.1002/eji.1830271110. PMID 9394803.
- Borgatti P, Zauli G, Cantley LC, Capitani S (1998). "Extracellular HIV-1 Tat protein induces a rapid and selective activation of protein kinase C (PKC)-alpha, and -epsilon and -zeta isoforms in PC12 cells". Biochem. Biophys. Res. Commun. 242 (2): 332–337. doi:10.1006/bbrc.1997.7877. PMID 9446795.
- Zhang J, Banfić H, Straforini F, et al. (1998). "A type II phosphoinositide 3-kinase is stimulated via activated integrin in platelets. A source of phosphatidylinositol 3-phosphate". J. Biol. Chem. 273 (23): 14081–14084. doi:10.1074/jbc.273.23.14081. PMID 9603905.
- Milani D, Mazzoni M, Zauli G, et al. (1998). "HIV-1 Tat induces tyrosine phosphorylation of p125FAK and its association with phosphoinositide 3-kinase in PC12 cells". AIDS. 12 (11): 1275–1284. doi:10.1097/00002030-199811000-00008. PMID 9708406.
- Jauliac S, Mazerolles F, Jabado N, et al. (1998). "Ligands of CD4 inhibit the association of phospholipase Cgamma1 with phosphoinositide 3 kinase in T cells: regulation of this association by the phosphoinositide 3 kinase activity". Eur. J. Immunol. 28 (10): 3183–3191. doi:10.1002/(SICI)1521-4141(199810)28:10<3183::AID-IMMU3183>3.0.CO;2-A. PMID 9808187.
- Brown RA, Domin J, Arcaro A, et al. (1999). "Insulin activates the alpha isoform of class II phosphoinositide 3-kinase". J. Biol. Chem. 274 (21): 14529–14532. doi:10.1074/jbc.274.21.14529. PMID 10329640.
- Idriss H, Kawa S, Damuni Z, et al. (2000). "HIV-1 reverse transcriptase is phosphorylated in vitro and in a cellular system". Int. J. Biochem. Cell Biol. 31 (12): 1443–1452. doi:10.1016/S1357-2725(99)00097-7. PMID 10641798.
- Domin J, Gaidarov I, Smith ME, et al. (2000). "The class II phosphoinositide 3-kinase PI3K-C2alpha is concentrated in the trans-Golgi network and present in clathrin-coated vesicles". J. Biol. Chem. 275 (16): 11943–11950. doi:10.1074/jbc.275.16.11943. PMID 10766823.
- Arcaro A, Zvelebil MJ, Wallasch C, et al. (2000). "Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors". Mol. Cell. Biol. 20 (11): 3817–3830. doi:10.1128/MCB.20.11.3817-3830.2000. PMC 85707. PMID 10805725.
- Park IW, Wang JF, Groopman JE (2001). "HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes". Blood. 97 (2): 352–358. doi:10.1182/blood.V97.2.352. PMID 11154208.
- Zauli G, Milani D, Mirandola P, et al. (2001). "HIV-1 Tat protein down-regulates CREB transcription factor expression in PC12 neuronal cells through a phosphatidylinositol 3-kinase/AKT/cyclic nucleoside phosphodiesterase pathway". FASEB J. 15 (2): 483–491. doi:10.1096/fj.00-0354com. PMID 11156964.
- Gaidarov I, Smith ME, Domin J, Keen JH (2001). "The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking". Mol. Cell. 7 (2): 443–449. doi:10.1016/S1097-2765(01)00191-5. PMID 11239472.
- Caldwell GM, Eddy RL, Day CD, et al. (2001). "Mapping of genes and transcribed sequences in a gene rich 400-kb region on human chromosome 11p15.1→p14". Cytogenet. Cell Genet. 92 (1–2): 103–107. doi:10.1159/000056877. PMID 11306805.
- Didichenko SA, Thelen M (2002). "Phosphatidylinositol 3-kinase c2alpha contains a nuclear localization sequence and associates with nuclear speckles". J. Biol. Chem. 276 (51): 48135–42. doi:10.1074/jbc.M104610200. PMID 11606566.
- Deregibus MC, Cantaluppi V, Doublier S, et al. (2002). "HIV-1-Tat protein activates phosphatidylinositol 3-kinase/ AKT-dependent survival pathways in Kaposi's sarcoma cells". J. Biol. Chem. 277 (28): 25195–25202. doi:10.1074/jbc.M200921200. PMID 11994280.
- Cook JA, August A, Henderson AJ (2002). "Recruitment of phosphatidylinositol 3-kinase to CD28 inhibits HIV transcription by a Tat-dependent mechanism". J. Immunol. 169 (1): 254–60. doi:10.4049/jimmunol.169.1.254. PMID 12077252.