Ozenoxacin
 | |
| Clinical data | |
|---|---|
| Trade names | Ozanex; Xepi |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
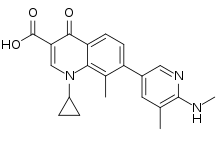
| Formula | C21H21N3O3 |
| Molar mass | 363.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ozenoxacin (INN; trade names Ozaenex and Xepi) is a quinolone antibiotic being approved for the treatment of impetigo.[1] A 1% topical cream is approved for treatment of impetigo in Canada[2] and in the United States.[3]
Ozenoxacin is active against some bacteria that have developed resistance to currently used quinolone and fluoroquinolone antibiotics.[4]
References
- ↑ "Ferrer successfully completes a phase III clinical trial in adult and paediatric patients with impetigo for novel antibacterial compound Ozenoxacin". drugs.com. June 2013.
- ↑ "Cipher Pharmaceuticals Receives Health Canada Approval of OZANEX (ozenoxacin cream 1%)" (Press release). Cipher Pharmaceuticals Inc.
- ↑ "Medimetriks Pharmaceuticals, Inc. Receives FDA Approval for Xepi™ (ozenoxacin) Cream, 1%, a Novel Topical Antibiotic for Impetigo" (Press release). Medimetriks Pharmaceuticals, Inc.
- ↑ López Y, Tato M, Espinal P, Garcia-Alonso F, Gargallo-Viola D, Cantón R, Vila J (Dec 2013). "In vitro activity of Ozenoxacin against quinolone-susceptible and quinolone-resistant gram-positive bacteria". Antimicrob Agents Chemother. 57 (12): 6389–6392. doi:10.1128/AAC.01509-13. PMC 3837899. PMID 24080666.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.