Isotopes of oxygen
| ||||||||||||||||||||||||||||||
| Standard atomic weight (Ar, standard) |
| |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
There are three known stable isotopes of oxygen (8O): 16O, 17O, and 18O.
Radioactive isotopes with mass numbers from 12O to 24O have also been characterized, all short-lived, with the longest-lived being 15O with a half-life of 122.24 seconds, while the shortest-lived isotope is 12O with a half-life of 580(30)×10−24 second.
Stable isotopes
Naturally occurring oxygen is composed of three stable isotopes, 16O, 17O, and 18O, with 16O being the most abundant (99.762% natural abundance). Depending on the terrestrial source, the standard atomic weight varies within the range of [15.99903, 15.99977] (the conventional value is 15.999). Known oxygen isotopes range in mass number from 12 to 24.
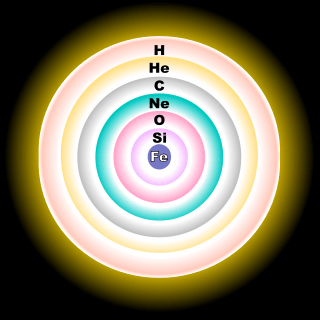
The relative and absolute abundance of 16O is high because it is a principal product of stellar evolution and because it is a primary isotope, meaning it can be made by stars that were initially made exclusively of hydrogen.[2] Most 16O is synthesized at the end of the helium fusion process in stars; the triple-alpha reaction creates 12C, which captures an additional 4He to make 16O. The neon burning process creates additional 16O.[2]
Both 17O and 18O are secondary isotopes, meaning that their nucleosynthesis requires seed nuclei. 17O is primarily made by the burning of hydrogen into helium during the CNO cycle, making it a common isotope in the hydrogen burning zones of stars.[2] Most 18O is produced when 14N (made abundant from CNO burning) captures a 4He nucleus, making 18O common in the helium-rich zones of stars.[2] Approximately a billion degrees Celsius is required for two oxygen nuclei to undergo nuclear fusion to form the heavier nucleus of sulfur.[3]
Measurements of the ratio of oxygen-18 to oxygen-16 are often used to interpret changes in paleoclimate. The isotopic composition of oxygen atoms in the Earth's atmosphere is 99.759% 16O, 0.037% 17O and 0.204% 18O.[4] Because water molecules containing the lighter isotope are slightly more likely to evaporate and fall as precipitation,[5] fresh water and polar ice on earth contains slightly less (0.1981%) of the heavy isotope 18O than air (0.204%) or seawater (0.1995%). This disparity allows analysis of temperature patterns via historic ice cores.
An atomic mass of 16 was assigned to oxygen prior to the definition of the unified atomic mass unit based upon 12C.[6] Since physicists referred to 16O only, while chemists meant the naturally-abundant mixture of isotopes, this led to slightly different mass scales between the two disciplines.
Radioisotopes
Fourteen radioisotopes have been characterized, with the most stable being 15O with a half-life of 122.24 s and 14O with a half-life of 70.606 s.[7] All of the remaining radioactive isotopes have half-lives that are less than 27 s and the majority of these have half-lives that are less than 83 milliseconds (ms).[7] For example, 24O has a half-life of 61 ms.[8] The most common decay mode for isotopes lighter than the stable isotopes is β+ decay (to nitrogen)[9][10][11] and the most common mode after is β− decay (to fluorine).
Oxygen-13
Oxygen-13 is an unstable isotope of oxygen. It consists of 8 protons and electrons, and 5 neutrons. It has a spin of 3/2-, and a half-life of 8.58 ms. Its atomic mass is 13.0248 Da. It decays to nitrogen-13 by electron capture, and has a decay energy of 17.765 MeV.[12] Its parent nuclide is fluorine-14.[13]
Oxygen-15
Oxygen-15 is an isotope of oxygen, frequently used in positron emission tomography, or PET imaging. It can be used, amongst other applications, in water for PET myocardial perfusion imaging and for brain imaging.[14][15] It has 8 protons, 7 neutrons, and 8 electrons. The total atomic mass is 15.0030654 amu. It has a half-life of 122.24 seconds.[16] Oxygen-15 is synthesized through deuteron bombardment of nitrogen-14 using a cyclotron.[17]
Oxygen-15 and nitrogen-13 are produced in the atmosphere when gamma rays (for example from lightning) knock neutrons out of oxygen-16 and nitrogen-14:[18]
- 16O + γ → 15O + n
- 14N + γ → 13N + n
The oxygen-15 decays with a half-life of about two minutes to nitrogen-15, emitting a positron. The positron quickly annihilates with an electron, producing two gamma rays of about 511 keV. After a lightning bolt, this gamma radiation dies down with a half-life of two minutes, but these low-energy gamma rays go on average only about 90 metres through the air. Together with rays produced from positrons from nitrogen-13 they may only be detected for a minute or so as the "cloud" of 15O and 13N floats by, carried by the wind.[19]
List of isotopes
| nuclide symbol |
Z(p) | N(n) | isotopic mass (u) |
half-life | decay mode(s)[20] | daughter isotope(s)[n 1] |
nuclear spin and parity |
representative isotopic composition (mole fraction) |
range of natural variation (mole fraction) |
|---|---|---|---|---|---|---|---|---|---|
| 12O | 8 | 4 | 12.034405(20) | 580(30)×10−24 s [0.40(25) MeV] |
2p (60.0%) | 10C | 0+ | ||
| p (40.0%) | 11N | ||||||||
| 13O | 8 | 5 | 13.024812(10) | 8.58(5) ms | β+ (89.1%) | 13N | (3/2−) | ||
| β+, p (10.9%) | 12C | ||||||||
| 14O | 8 | 6 | 14.00859625(12) | 70.598(18) s | β+ | 14N | 0+ | ||
| 15O | 8 | 7 | 15.0030656(5) | 122.24(16) s | β+ | 15N | 1/2− | ||
| 16O[n 2] | 8 | 8 | 15.99491461956(16) | Stable | 0+ | 0.99757(16) | 0.99738–0.99776 | ||
| 17O[n 3] | 8 | 9 | 16.99913170(12) | Stable | 5/2+ | 3.8(1)×10−4 | 3.7×10−4–4.0×10−4 | ||
| 18O[n 2][n 4] | 8 | 10 | 17.9991610(7) | Stable | 0+ | 2.05(14)×10−3 | 1.88×10−3–2.22×10−3 | ||
| 19O | 8 | 11 | 19.003580(3) | 26.464(9) s | β− | 19F | 5/2+ | ||
| 20O | 8 | 12 | 20.0040767(12) | 13.51(5) s | β− | 20F | 0+ | ||
| 21O | 8 | 13 | 21.008656(13) | 3.42(10) s | β− | 21F | (1/2,3/2,5/2)+ | ||
| 22O | 8 | 14 | 22.00997(6) | 2.25(15) s | β− (78.0%) | 22F | 0+ | ||
| β−, n (22.0%) | 21F | ||||||||
| 23O | 8 | 15 | 23.01569(13) | 82(37) ms | β−, n (57.99%) | 22F | 1/2+# | ||
| β− (42.0%) | 23F | ||||||||
| 24O | 8 | 16 | 24.02047(25) | 65(5) ms | β−, n (57.99%) | 23F | 0+ | ||
| β− (42.01%) | 24F | ||||||||
Oxygen isotopes with 25–28 nucleons exist for less than 1 microsecond, since they are beyond the neutron drip line.[21]
Notes
- The precision of the isotope abundances and atomic mass is limited through variations. The given ranges should be applicable to any normal terrestrial material.
- Values marked # are not purely derived from experimental data, but at least partly from systematic trends. Spins with weak assignment arguments are enclosed in parentheses.
- Uncertainties are given in concise form in parentheses after the corresponding last digits. Uncertainty values denote one standard deviation, except isotopic composition and standard atomic mass from IUPAC, which use expanded uncertainties.
- Nuclide masses are given by IUPAP Commission on Symbols, Units, Nomenclature, Atomic Masses and Fundamental Constants (SUNAMCO).
- Isotope abundances are given by IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW).
See also
Notes and references
- ↑ Meija, J.; et al. (2016). "Atomic weights of the elements 2013 (IUPAC Technical Report)". Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- 1 2 3 4 B. S. Meyer (September 19–21, 2005). "Nucleosynthesis and galactic chemical evolution of the isotopes of oxygen" (PDF). Proceedings of the NASA Cosmochemistry Program and the Lunar and Planetary Institute. Workgroup on Oxygen in the Earliest Solar System. Gatlinburg, Tennessee. 9022.
- ↑ Emsley 2001, p. 297.
- ↑ Cook 1968, p. 500.
- ↑ Dansgaard, W (1964). "Stable isotopes in precipitation" (PDF). Tellus. 16: 436–468. Bibcode:1964TellA..16..436D. doi:10.1111/j.2153-3490.1964.tb00181.x.
- ↑ Parks & Mellor 1939, Chapter VI, Section 7.
- 1 2 K. L. Barbalace. "Periodic Table of Elements: O - Oxygen". EnvironmentalChemistry.com. Retrieved 2007-12-17.
- ↑ Ekström, L. P.; Firestone, R. B. (28 February 1999). "Oxygen-24". WWW Table of Radioactive Isotopes. LUNDS Universitet, LBNL Isotopes Project. Archived from the original on 13 August 2009. Retrieved 2009-06-08.
- ↑ "NUDAT". Retrieved 2009-07-06.
- ↑ "NUDAT". Retrieved 2009-07-06.
- ↑ "NUDAT". Retrieved 2009-07-06.
- ↑ "Periodic Table of Elements: O - Oxygen". EnvironmentalChemistry.com. 1995-10-22. Retrieved 2014-12-02.
- ↑ "Periodic Table of Elements: F - Fluorine". EnvironmentalChemistry.com. 1995-10-22. Retrieved 2014-12-02.
- ↑ Rischpler, Christoph; Higuchi, Takahiro; Nekolla, Stephan G. (22 November 2014). "Current and Future Status of PET Myocardial Perfusion Tracers". Current Cardiovascular Imaging Reports. 8 (1). doi:10.1007/s12410-014-9303-z. PMC 4333146.
- ↑ Kim, E. Edmund; Lee, Myung-Chul; Inoue, Tomio; Wong, Wai-Hoi (2012). Clinical PET and PET/CT: Principles and Applications. Springer. p. 182. ISBN 9781441908025.
- ↑ "oxygen 15 - definition of oxygen 15 by Medical dictionary". Medical-dictionary.thefreedictionary.com. Retrieved 2014-12-02.
- ↑ "Production of PET Radionuclides". Austin Hospital, Austin Health. Archived from the original on 15 January 2013. Retrieved 6 December 2012.
- ↑ Timmer, John (25 November 2017). "Lightning strikes leave behind a radioactive cloud". Ars Technica.
- ↑ Teruaki Enoto; et al. (Nov 23, 2017). "Photonuclear reactions triggered by lightning discharge". Nature. Bibcode:2017Natur.551..481E. doi:10.1038/nature24630.
- ↑ "Universal Nuclide Chart". Nucleonica. Retrieved 2014-12-02. (Registration required (help)).
- ↑ "Nuclear Physicists Examine Oxygen's Limits". Science Daily. 2007-09-18. Retrieved 2010-09-16.
References
- For the table
- Isotope masses from the area:
- G. Audi; A. H. Wapstra; C. Thibault; J. Blachot; O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001. Archived from the original (PDF) on 2008-09-23.
- Isotopic compositions and standard atomic masses from:
- J. R. de Laeter; J. K. Böhlke; P. De Bièvre; H. Hidaka; H. S. Peiser; K. J. R. Rosman; P. D. P. Taylor (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)" (PDF). Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
- M. E. Wieser (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry. 78 (11): 2051–2066. doi:10.1351/pac200678112051. Lay summary.
- Half-life, spin, and isomer data selected from the following sources. See editing notes on this article's talk page.
- G. Audi; A. H. Wapstra; C. Thibault; J. Blachot; O. Bersillon (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001. Archived from the original (PDF) on 2008-09-23.
- National Nuclear Data Center. "NuDat 2.1 database". Brookhaven National Laboratory. Retrieved September 2005. Check date values in:
|accessdate=(help) - N. E. Holden (2004). "Table of the Isotopes". In D. R. Lide. CRC Handbook of Chemistry and Physics (85th ed.). CRC Press. Section 11. ISBN 978-0-8493-0485-9.
- For the prose
- Cook, Gerhard A.; Lauer, Carol M. (1968). "Oxygen". In Clifford A. Hampel. The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 499–512. LCCN 68-29938.
- Emsley, John (2001). "Oxygen". Nature's Building Blocks: An A–Z Guide to the Elements. Oxford, England, UK: Oxford University Press. pp. 297–304. ISBN 0-19-850340-7.
- Parks, G. D.; Mellor, J. W. (1939). Mellor's Modern Inorganic Chemistry (6th ed.). London: Longmans, Green and Co.