Oxidative coupling
Oxidative coupling in chemistry is a coupling reaction of two molecular entities through an oxidative process. Usually oxidative couplings are catalysed by a transition metal complex. Many such couplings utilizedioxygen as the stoichiometric oxidant but proceed by electron transfer.[1]
C-C Couplings
Many oxidative couplings generate new C-C bonds. Early examples involve coupling of terminal alkynes:[2]
- 2 RC≡CH + 2 Cu(I) → RC≡C-C≡CR + 2 Cu + 2 H+
Coupling of methane
Coupling reactions involving methane are highly sought, related to C1 chemistry because C2 derivatives are far more valuable than methane. The oxidative coupling of methane gives ethylene:[3][4]
- 2CH
4 + O
2 → C
2H
4 + 2H
2O
Aromatic coupling
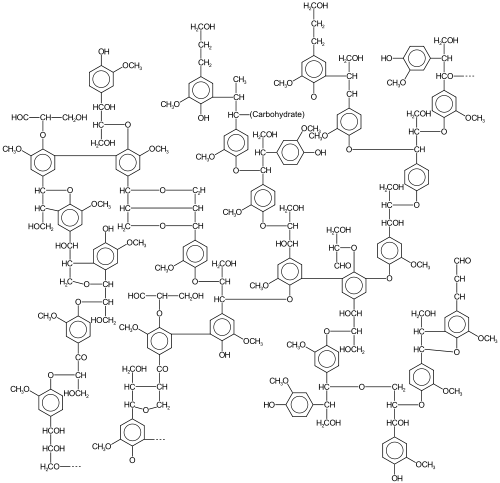
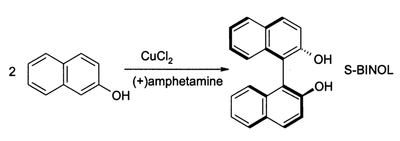
In oxidative aromatic coupling the reactants are electron-rich aromatic compounds. Typical substrates are phenols and typical catalysts are copper and iron compounds and enzymes.[6] The first reported synthetic application dates back to 1868 with Julius Löwe and the synthesis of ellagic acid by heating gallic acid with arsenic acid or silver oxide.[7] Another reaction is the synthesis of 1,1'-Bi-2-naphthol from 2-naphthol by iron chloride, discovered in 1873 by Alexander Dianin [8] (S)-BINOL can be prepared directly from an asymmetric oxidative coupling of 2-naphthol with copper(II) chloride.[9]
Other oxidative couplings
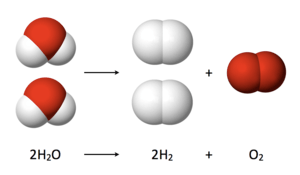
The oxygen evolution reaction entails, in effect, the oxidative coupling of water molecules to give O2.
References
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). doi:10.1351/goldbook
- ↑ Alison E. Wendlandt, Alison M. Suess, Shannon S. Stahl (2011). "Copper‐Catalyzed Aerobic Oxidative C-H Functionalizations: Trends and Mechanistic Insights". Angew. Chem. Int. Ed. 50: 11062–11087. doi:10.1002/anie.201103945.
- ↑ Zhang, Q. (2003). "Recent Progress in Direct Partial Oxidation of Methane to Methanol". J. Natural Gas Chem. 12: 81–89.
- ↑ Olah, G., Molnar, A. “Hydrocarbon Chemistry” John Wiley & Sons, New York, 2003. ISBN 978-0-471-41782-8.
- ↑ Lebo, Stuart E. Jr.; Gargulak, Jerry D.; McNally, Timothy J. (2001). "Lignin". Kirk-Othmer Encyclopedia of Chemical Technology. Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.12090714120914.a01.pub2. ISBN 0-471-23896-1. Retrieved 2007-10-14.
- ↑ Grzybowski, M., Skonieczny, K., Butenschön, H. and Gryko, D. T. (2013), Comparison of Oxidative Aromatic Coupling and the Scholl Reaction Angew. Chem. Int. Ed., 52: 9900–9930. doi:10.1002/anie.201210238
- ↑ Löwe, Zeitschrift für Chemie, 1868, 4, 603
- ↑ A. P. Dianin, Zh. Russ. Fiz.-Khim. O-va. 1874 , 183
- ↑ Brussee, J.; Jansen, A. C. A. (1983). "A highly stereoselective synthesis of S-(−)-[1,1′-binaphthalene]-2,2′-diol". Tetrahedron Letters. 24 (31): 3261–3262. doi:10.1016/S0040-4039(00)88151-4.