Overman rearrangement
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate.[1][2][3] The Overman rearrangement was discovered in 1974 by Larry Overman.[4]
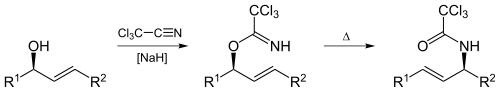
The [3,3]-sigmatropic rearrangement is diastereoselective and requires heating or the use of Hg(II) or Pd(II) salts as catalysts.[5] The resulting allylamine structures can be transformed into many chemically and biologically important natural and un-natural amino acids (like (1-adamantyl)glycine).[6]
The Overman rearrangement may also be used for asymmetric synthesis.[7][8]
See also
References
- ↑ Larry E. Overman (1976). "A general method for the synthesis of amines by the rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions". J. Am. Chem. Soc. 98 (10): 2901–2910. doi:10.1021/ja00426a038.
- ↑ Overman, L. E. (1980). "Allylic and propargylic imidic esters in organic synthesis". Accounts of Chemical Research. 13 (7): 218–224. doi:10.1021/ar50151a005.
- ↑ Organic Syntheses, Coll. Vol. 6, p.507; Vol. 58, p.4 (Article)
- ↑ Overman, L. E. (1974). "Thermal and mercuric ion catalyzed [3,3]-sigmatropic rearrangement of allylic trichloroacetimidates. 1,3 Transposition of alcohol and amine functions". Journal of the American Chemical Society. 96 (2): 597–599. doi:10.1021/ja00809a054.
- ↑ Overman, L. E.; Carpenter, N. E. Org. React. 2005, 66, 1. doi:10.1002/0471264180.or066.01
- ↑ Chen, Y. K.; Lurain, A. E.; Walsh, P. J. (2002). "A General, Highly Enantioselective Method for the Synthesis of D and L α-Amino Acids and Allylic Amines". Journal of the American Chemical Society. 124 (41): 12225–12231. doi:10.1021/ja027271p. PMID 12371863.
- ↑ Anderson, C. E.; Overman, L. E. J. Am. Chem. Soc. 2003, 125, 12412–12413. (doi:10.1021/ja037086r)
- ↑ Asymmetric Overman Rearrangement Organic Syntheses, Vol. 82, p.134 (2005). (Article)
Further reading
- Isobe, M. et al. Tetrahedron Lett. 1990, 31, 3327.
- Allmendinger, T. et al. Tetrahedron Letters 1990, 31, 7301.
- Nishikawa, T.; Asai, M.; Ohyabu, N.; Isobe, M.; J. Org. Chem. 1998, 63(1), 188-192. PMID 11674062
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.