Nitrogen inversion
 Nitrogen inversion in ammonia | ||
 |
⇌ | 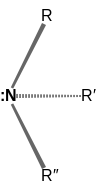 |
| Inversion of an amine. The C3 axis of the amine is presented as horizontal, and the pair of dots represent the lone pair of the nitrogen atom collinear with that axis. A mirror plane can be imagined to relate the two amine molecules on either side of the arrows. If the three R groups attached to the nitrogen are all unique, then the amine is chiral; whether it can be isolated depends on the free energy required for the molecule's inversion. | ||
In chemistry, nitrogen inversion is a fluxional process in nitrogen and amines, whereby the molecule "turns inside out". It is a rapid oscillation of the nitrogen atom and substituents, the nitrogen "moving" through the plane formed by the substituents (although the substituents also move - in the other direction);[1] the molecule passing through a planar transition state.[2] For a compound that would otherwise be chiral due to a nitrogen stereocenter, nitrogen inversion provides a low energy pathway for racemization, usually making chiral resolution impossible.[3]
Nitrogen inversion is one case of the more general phenomenon of pyramidal inversion, which applies to carbanions, phosphines, arsines, stibines, and sulfoxides.[4][5]
Energy barrier
The ammonia interconversion is rapid at room temperature, inverting 30 billion times per second. Two factors contribute to the rapidity of the inversion: a low energy barrier (24.2 kJ/mol; 5.8 kcal/mol) and a narrow width of the barrier itself, which allows for frequent quantum tunnelling (see below). In contrast, phosphine (PH3) inverts very slowly at room temperature (energy barrier: 132 kJ/mol).[6]
Quantum effects
Ammonia exhibits a quantum tunnelling due to a narrow tunneling barrier,[7] and not due to thermal excitation. Superposition of two states leads to energy level splitting, which is used in ammonia masers.
Examples
The inversion of ammonia was first detected by microwave spectroscopy in 1934.[8]
In one study the inversion in an aziridine was slowed by a factor of 50 by placing the nitrogen atom in the vicinity of a phenolic alcohol group compared to the oxidized hydroquinone.[9]
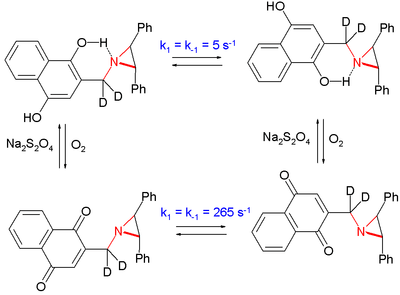
The system interconverts by oxidation by oxygen and reduction by sodium dithionite.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 423. ISBN 0-08-037941-9.
- ↑ J. M. Lehn (1970). "Nitrogen Inversion: Experiment and Theory". Fortschr. Chem. Forsch. 15: 311–377. doi:10.1007/BFb0050820.
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, pp. 142–145, ISBN 0-471-72091-7
- ↑ Arvi Rauk, Leland C. Allen, Kurt Mislow (1970). "Pyramidal Inversion". Angew. Chem. Int. Ed. 9: 400–414. doi:10.1002/anie.197004001.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Pyramidal inversion".
- ↑ Kölmel, C.; Ochsenfeld, C.; Ahlrichs, R. (1991). "An ab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines". Theor. Chim. Acta. 82 (3–4): 271–284. doi:10.1007/BF01113258.
- ↑ Feynman, Richard P.; Robert Leighton; Matthew Sands (1965). "The Hamiltonian matrix". The Feynman Lectures on Physics. Volume III. Massachusetts, USA: Addison-Wesley. ISBN 0-201-02118-8.
- ↑ Cleeton, C.E.; Williams, N.H. (1934). "Electromagnetic waves of 1.1 cm wave-length and the absorption spectrum of ammonia". Physical Review. 45 (4): 234–237. Bibcode:1934PhRv...45..234C. doi:10.1103/PhysRev.45.234.
- ↑ Control of Pyramidal Inversion Rates by Redox Switching Mark W. Davies, Michael Shipman, James H. R. Tucker, and Tiffany R. Walsh J. Am. Chem. Soc.; 2006; 128(44) pp. 14260–14261; (Communication) doi:10.1021/ja065325f