Nef isocyanide reaction
The Nef isocyanide reaction is an addition reaction that takes place between isocyanides and acyl chlorides to form imidoyl chloride products, a process first discovered by John Ulrich Nef.[1][2] The reaction should not be confused with the better known Nef reaction or the Nef synthesis, which were discovered by the same chemist.
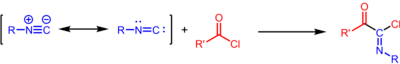
The product imidoyl chloride can be hydrolyzed to give the amide, trapped with other nucleophiles, or undergo halide abstraction with silver salts to form an acyl nitrilium intermediate.[3]
The reaction is of some theoretical interest, as kinetic measurements[4] and DFT studies[5] have indicated that the addition occurs in one step, without the intermediacy of a tetrahedral intermediate that is commonly proposed for carbonyl addition reactions.
See also
References
- ↑ Nef, J. U. (1892). "Ueber das zweiwerthige Kohlenstoffatom". Justus Liebig's Annalen der Chemie. 270 (3): 267–335. doi:10.1002/jlac.18922700302. ISSN 0075-4617.
- ↑ Tron, Gian; El Kaïm, Laurent; La Spisa, Fabio (2014-03-05). "The Nef Reaction of Isocyanides". Synthesis. 46 (07): 829–841. doi:10.1055/s-0033-1338596. ISSN 0039-7881.
- ↑ Westling, Mark; Smith, Richard; Livinghouse, Tom (April 1986). "A convergent approach to heterocycle synthesis via silver ion mediated .alpha.-ketoimidoyl halide-arene cyclizations. An application to the synthesis of the erythrinane skeleton". The Journal of Organic Chemistry. 51 (8): 1159–1165. doi:10.1021/jo00358a001. ISSN 0022-3263.
- ↑ Ugi, Ivar; Fetzer, Uwe (April 1961). "Isonitrile, III. Die Addition von Carbonsäurechloriden an Isonitrile". Chemische Berichte (in German). 94 (4): 1116–1121. doi:10.1002/cber.19610940433. ISSN 0009-2940.
- ↑ Chéron, Nicolas; El Kaïm, Laurent; Grimaud, Laurence; Fleurat-Lessard, Paul (2011-09-08). "A Density Functional Theory Study of the Nef-Isocyanide Reaction: Mechanism, Influence of Parameters and Scope". The Journal of Physical Chemistry A. 115 (35): 10106–10112. doi:10.1021/jp205909d. ISSN 1089-5639.