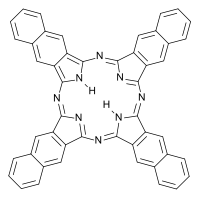
Naphthalocyanine
 | |
| Names | |
|---|---|
| IUPAC name
(2Z,15Z,27Z,41Z)-2,15,28,41,53,54,55,56-octaazatridecacyclo[40.10.1.13,14.116,27.129,40.04,13.06,11.017,26.019,24.030,39.032,37.043,52.045,50]hexapentaconta-1(53),2,4,6,8,10,12,14(56),15,17,19,21,23,25,27,29,31,33,35,37,39,41,43,45,47,49,51-heptacosaene (non-preferred name) | |
| Other names
Tetrabenzo[g]quinoxalino-2,3-porphyrazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C48H26N8 | |
| Molar mass | 714.79 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Naphthalocyanine is a derivative of phthalocyanine used as a component in the development of IBM's single-molecule logic switch.[1]
It was used to show the first images of charge distribution over a single molecule in November 2011.[2][3]
Its derivatives may also have uses in photodynamic cancer treatment.[4]
References
- ↑ Liljeroth, P.; Repp, J.; Meyer, G. (2007). "Current-Induced Hydrogen Tautomerization and Conductance Switching of Naphthalocyanine Molecules". Science. 317 (5842): 1203–1206. doi:10.1126/science.1144366. PMID 17761878.
- ↑ Mohn, F.; Gross, L.; Moll, N.; Meyer, G. (2012). "Imaging the charge distribution within a single molecule". Nature Nanotechnology. doi:10.1038/NNANO.2012.20.
- ↑ "Scientists image the charge distribution within a single molecule for the first time". Physorg.com. 2012-02-27. Retrieved 2012-02-27.
- ↑ Shopova, M.; Woehrle, D.; Mantareva, V.; Mueller, S. (1999). "Naphthalocyanine Complexes as Potential Photosensitizers for Photodynamic Therapy of Tumors". Journal of Biomedical Optics. 4 (3): 276. doi:10.1117/1.429930.
External links
- Timmer, J. (2007) Storing data in molecules: shifting atoms and flipping bits, ars technica online [accessed 8 September 2007]
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.