Myclobutanil
 | |
| Names | |
|---|---|
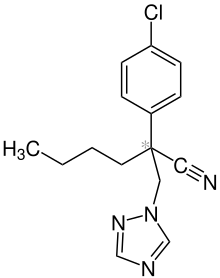
| IUPAC name
2-(4-Chlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)hexanenitrile | |
| Identifiers | |
3D model (JSmol) |
|
| 7138849 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.101.000 |
| EC Number | 410-400-0 |
| KEGG | |
| MeSH | Systhane |
PubChem CID |
|
| RTECS number | XZ5257000 |
| UN number | 3077 |
| |
| |
| Properties | |
| C15H17ClN4 | |
| Molar mass | 288.78 g·mol−1 |
| Appearance | Pale, yellow, translucent crystals |
| Melting point | 63 to 68 °C (145 to 154 °F; 336 to 341 K) |
| Boiling point | 202 to 208 °C (396 to 406 °F; 475 to 481 K) at 130 Pa |
| 142 mg⋅dm−3 | |
| Hazards | |
| GHS pictograms |    |
| GHS signal word | Warning |
| H302, H319, H361, H411 | |
| P273, P281, P305+351+338 | |
| NFPA 704 | |
| Flash point | > 100 °C (212 °F; 373 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Myclobutanil is a triazole chemical used as a fungicide.[1] It is a steroid demethylation inhibitor, specifically inhibiting ergosterol biosynthesis.[2] Ergosterol is a critical component of fungal cell membranes. When heated, myclobutanil decomposes to produce corrosive and/or toxic fumes, including carbon monoxide, carbon dioxide, hydrogen chloride, hydrogen cyanide, and nitrogen oxides.[3][4] Myclobutanil is banned in Canada, Colorado, Washington, and Oregon for the production of medical and recreational marijuana; however, despite the Canadian ban, myclobutanil has been discovered in medical marijuana produced by at least one government licensed grower.[5]
Stereoisomerism
| Myclobutanil (2 stereoisomers) | |
|---|---|
-Myclobutanil_V1.svg.png) (S)-configuration |
-Myclobutanil_V1.svg.png) (R)-configuration |
References
- ↑ Myclobutanil, PAN Pesticides Database
- ↑ "Myclobutanil Product Sheet". Kingtai Chemicals Co.
- ↑ GOV, NOAA Office of Response and Restoration, US. "MYCLOBUTANIL - CAMEO Chemicals - NOAA". cameochemicals.noaa.gov.
- ↑ "Product Safety Assessment: Myclobutanil".
- ↑ "Canadians not told about banned pesticide found in medical pot supply" – via The Globe and Mail.
External links
- Myclobutanil in the Pesticide Properties DataBase (PPDB)
- International Programme on Chemical Safety
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.
