Molybdocene dihydride
 | |
| Names | |
|---|---|
| Other names
dihydridobis(cyclopentadienyl)molybdenum(IV) | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C10H12Mo | |
| Molar mass | 228.16 g·mol−1 |
| Appearance | yellow-brown powder |
| Melting point | 163–165 °C (325–329 °F; 436–438 K) |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
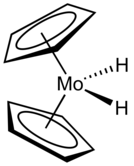
Molybdocene dihydride is the organomolybdenum compound with the formula (η5-C5H5)2MoH2. Commonly abbreviated as Cp2MoH2, it is a yellow air--sensitive solid that dissolves in some organic solvents.
The compound is prepared by combining molybdenum pentachloride, sodium cyclopentadienide, and sodium borohydride.[1][2] The dihydride converts to molybdocene dichloride upon treatment with chloroform:
The compound adopts a "clamshell" structure where the Cp rings are not parallel.[3]
References
- ↑ Silavwe, Ned D.; Castellani, Michael P.; Tyler, David R. (1992). "Bis(η5-Cyclopentadienyl)Molybdenum(IV) Complexes". Inorganic Syntheses. 29: 204–211. doi:10.1002/9780470132609.ch50.
- ↑ Green, M. L. H.; McCleverty, J. A.; Pratt, L.; Wilkinson, G. (1961). "The di-π-cyclopentadienyl hydrides of tantalum, molybdenum, and tungsten". Journal of the Chemical Society: 4854–9. doi:10.1039/JR9610004854.
- ↑ K. Prout, T. S. Cameron, R. A. Forder, and in parts S. R. Critchley, B. Denton and G. V. Rees "The crystal and molecular structures of bent bis-π-cyclopentadienyl-metal complexes: (a) bis-π-cyclopentadienyldibromorhenium(V) tetrafluoroborate, (b) bis-π-cyclopentadienyldichloromolybdenum(IV), (c) bis-π-cyclopentadienylhydroxomethylaminomolybdenum(IV) hexafluorophosphate, (d) bis-π-cyclopentadienylethylchloromolybdenum(IV), (e) bis-π-cyclopentadienyldichloroniobium(IV), (f) bis-π-cyclopentadienyldichloromolybdenum(V) tetrafluoroborate, (g) μ-oxo-bis[bis-π-cyclopentadienylchloroniobium(IV)] tetrafluoroborate, (h) bis-π-cyclopentadienyldichlorozirconium" Acta Crystallogr. 1974, volume B30, pp. 2290–2304. doi:10.1107/S0567740874007011
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.