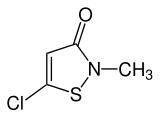
Methylchloroisothiazolinone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5-Chloro-2-methyl-1,2-thiazol-3(2H)-one | |
| Other names
5-Chloro-2-methylisothiazol-3(2H)-one 5-Chloro-2-methyl-4-isothiazolin-3-one Chloromethylisothiazolinone Chloromethylisothiazolone Methylchloroisothiazolinone Methylchloroisothiazolone CMI CMIT MCI MCIT | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.043.167 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C4H4ClNOS | |
| Molar mass | 149.59 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.02 g/cm³ |
| Miscible | |
| Hazards | |
| R-phrases (outdated) | R23/24/25 R34 R43 R50/53 |
| S-phrases (outdated) | (S2) S26 S28 S36/37/39 S45 S60 S61 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylchloroisothiazolinone, also referred to as MCI, is a preservative with antibacterial and antifungal effects within the group of isothiazolinones. It is effective against gram-positive and gram-negative bacteria, yeast, and fungi.
Methylchloroisothiazolinone is found in many water-based personal care products and cosmetics.[1] Methylchloroisothiazolinone was first used in cosmetics in the 1970s. It is also used in glue production, detergents, paints, fuels, and other industrial processes. Methylchloroisothiazolinone is known by the registered tradename Kathon CG when used in combination with methylisothiazolinone.[2]
Methylchloroisothiazolinone may be used in combination with other preservatives including ethylparaben, benzalkonium chloride, and bronopol.
Safety
In pure form or in high concentrations, methylchloroisothiazolinone is a skin and membrane irritant and causes chemical burns. In the United States, accepted concentrations are 15 ppm in rinse-offs and 8 ppm in other cosmetics. In Canada, the accepted concentrations are 15 ppm and 7.5 ppm respectively.[3] The Canadian levels are also reflected in a paper published in the International Journal of Toxicology.[4]
As of 2008, the International Agency for Research on Cancer (IARC), did not list methylchloroisothiazolinone as a known, probable, or possible human carcinogen,[5] nor have in vivo tests found evidence of carcinogenic activity.
Allergic contact dermatitis
Methylchloroisothiazolinone can cause allergic reactions in some people.[6] Since 2013, the use of this ingredient in cosmetics has received increased media coverage in the UK. Some cosmetics methylchloroisothiazolinone in have been investigated by the BBC programme Watchdog.[7] The first publication of the preservative as a contact allergen was in 1988.[8] Cases of photoaggravated allergic contact dermatitis, i.e. worsening of skin lesions after sun exposure, have also been reported.[6]
References
- ↑ Reinhard; et al. (2001). "Preservation of products with MCI/MI in Switzerland". Contact Dermatitis. 45 (5): 257–264. doi:10.1034/j.1600-0536.2001.450501.x. PMID 11722483.
- ↑ Knudsen BB, Menne T (1990). "Kathon CG--a new contact sensitizing preservative". Ugeskrift for Lægerer. 152 (10): 656–657. PMID 2321281.
- ↑ "Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients". Health Canada. Retrieved 24 February 2015.
- ↑ "6 Final Report on the Safety Assessment of Methylisothiazolinone and Methylchloroisothiazolinone". sagepub.com. International Journal of Toxicology. January 1992. pp. 75–128. doi:10.3109/10915819209141993. Retrieved 24 February 2015.
- ↑ "International Agency for Research on Cancer". IARC (updated November 2007). Retrieved 7 January 2008.
- 1 2 Pirmez R, Fernandes ALC, Melo MGM. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic? Br J Dermatol. 2015 Jun 30. doi: 10.1111/bjd.13986. [Epub ahead of print] http://onlinelibrary.wiley.com/doi/10.1111/
- ↑ "Archived copy". Archived from the original on 10 September 2014. Retrieved 8 June 2015.
- ↑ De Groot, A. C.; Weyland, J. W. (1988). "Kathon CG: A review". Journal of the American Academy of Dermatology. 18 (2 Pt 1): 350–358. doi:10.1016/s0190-9622(88)70051-1. PMID 3279090.