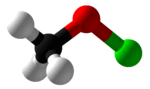
Methyl hypochlorite
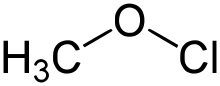 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Chlorooxy)methane | |||
| Systematic IUPAC name
Methyl hypochlorite | |||
| Other names
Hypochlorous acid methyl ester; Methoxy chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| CH3ClO | |||
| Molar mass | 66.48 g·mol−1 | ||
| Appearance | Gas | ||
| Odor | Pungent | ||
| Density | 1.058 g/cm3 | ||
| Melting point | −120.4 °C (−184.7 °F; 152.8 K) | ||
| Boiling point | 9.18 °C (48.52 °F; 282.33 K) | ||
| Decomposes | |||
Refractive index (nD) |
1.343 | ||
| Hazards | |||
| R-phrases (outdated) | R3 R8 R23/24/25 R35 | ||
| NFPA 704 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Methyl hypochlorite is the simplest of the organic alkyl hypochlorites. It is an explosively unstable and highly toxic compound that can be produced by the reaction of methanol with hypochlorous acid.[1] It was first synthesized by Sandmeyer in the 1880s.[2]
Methyl hypochlorite can form in the Earth's atmosphere by a reaction between ClO and CH3OO and is thought to be an important species in ozone destruction over the Artic and Antarctic regions.[3]
References
- ↑ Taylor, M. C.; MacMullin, R. B.; Gammal, C. A. (February 1925). "HYPOCHLOROUS ACID AND THE ALKYL HYPOCHLORITES". Journal of the American Chemical Society. 47 (2): 395–403. doi:10.1021/ja01679a017.
- ↑ Sandmeyer, Traugott (January 1886). "Ueber Aethyl- und Methylhypochlorit". Berichte der deutschen chemischen Gesellschaft. 19 (1): 857–861. doi:10.1002/cber.188601901196.
- ↑ Helleis, Frank; Crowley, John; Moortgat, Geert (15 August 1994). "Temperature dependent CH3OCl formation in the reaction between CH3O2 and ClO". Geophysical Research Letters. 21 (17): 1795–1798. doi:10.1029/94GL01280.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.