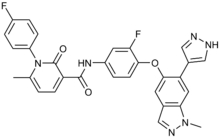
Merestinib
 | |
| Clinical data | |
|---|---|
| Routes of administration | PO |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C30H22F2N6O3 |
| Molar mass | 552.54 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Merestinib (LY2801653) is an experimental cancer drug in development by Eli Lilly. It is a small molecule inhibitor of MET and several other receptor tyrosine kinases such as MST1R, FLT3, AXL, MERTK, TEK, ROS1, NTRK1/2/3, and DDR1/2.
Meristinib is part of a phase II clinical trial for advanced billiary tract cancer. The study is expected to be complete in April 2018.[1] Phase II clinical trials for non-small cell lung cancer and solid tumors began in November 2016.[2]
References
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.