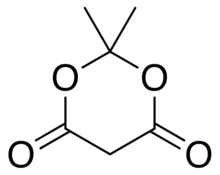
Meldrum's acid
 | |
| Names | |
|---|---|
| IUPAC name
2,2-Dimethyl-1,3-dioxane-4,6-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.016.358 |
PubChem CID |
|
| |
| |
| Properties | |
| C6H8O4 | |
| Molar mass | 144.13 g·mol−1 |
| Melting point | 94 to 95 °C (201 to 203 °F; 367 to 368 K) (decomposes)[1] |
| Acidity (pKa) | 4.97 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid.[2]
As an alternative to its original preparation, Meldrum's acid can be synthesized from malonic acid, isopropenyl acetate (an enol derivative of acetone), and catalytic sulfuric acid. Meldrum's acid has a high acidity with a pKa of 4.97. Like malonic acid and its ester derivatives, and other 1,3-dicarbonyl compounds, Meldrum's acid can easily be deprotonated and serve as a reactant for a variety of nucleophilic reactions.
Synthesis
Meldrum misidentified the structure of the chemical he discovered as a β-lactone of β-hydroxyisopropylmalonic acid.[3] Later analysis by others revealed that it was instead a but the structure was later found to be the 1,3-dioxane bislactone.

Reactivity
Ketene formation
Meldrum's acid displays thermal instability. At high temperatures, Meldrum's acid undergoes a pericyclic reaction that releases acetone and carbon dioxide and produces a highly reactive ketene compound.[4] Ketene intermediates can be isolated using flash vacuum pyrolysis (FVP) of Meldrum's acid at high temperatures. These highly electrophilic ketenes allow various addition reactions to take place by reacting the unstable ketene with other chemicals. Alternately, the pyrolysis can be performed in solution, allowing a one-pot reaction with chemicals already present rather than isolating the unstable ketene intermediate. With relative simplicity, is possible to form new C–C bonds, rings, amides, esters, and acids. The ability to form such diverse products makes Meldrum's acid a very useful reagent for synthetic chemists.[5]
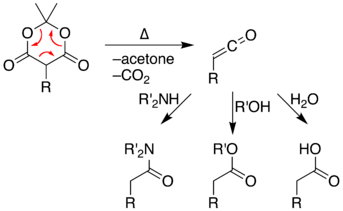
Alkylation and acylation
Meldrum's acid high acidity was long considered anomalous—it is 8 orders of magnitude more acidic than the closely related compound dimethyl malonate. In 2004, Ohwada and coworkers resolved the Meldrum acid anomaly by performing several calculations.[6] Ohwada noticed that the energy-minimizing conformation structure of the compound places the alpha proton's σCH orbital in the proper geometry to align with the π*CO, so that the ground state poses unusually strong destabilization of the C-H bond.
The position between the two carbonyl groups in Meldrum's acid is acidic, allowing simple alkylation and acylation at this position. For example, deprotonation and reaction with a simple alkyl halide allows formation of products containing carbon chains attached to the ring. Alternatively, this position can be acylated with a carboxylic acid chloride (RCOCl).
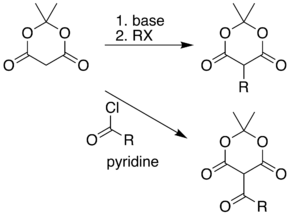
These two reactions allow Meldrum's acid to serve as a starting scaffold for the synthesis of many different structures with various functional groups. Paired with the pyrolysis and nucleophilic trapping reaction, above, the alkylated products can be carried on to produce a range of different amide and ester compounds. Heating the acyl product in the presence of an alcohol leads to ester exchange and decarboxylation in a process similar to the malonic ester synthesis. The reactive nature of the cyclic-diester allows good reactivity even for alcohols as hindered as t-butanol.[7] Ketoesters of this type are useful in the Knorr pyrrole synthesis.
References
- ↑ "Meldrum's Acid". The Merck Index. 14th. edition. Merck Research Laboratories. 2006. p. 1005. ISBN 978-0-911910-00-1.
- ↑ Meldrum, Andrew N. (1908). "A β-lactonic acid from acetone and malonic acid". J. Chem. Soc., Trans. 93: 598–601. doi:10.1039/CT9089300598.
- ↑ Davidson, David; Bernhard, Sidney A. (1948). "The Structure of Meldrum's Supposed β-Lactonic Acid". J. Am. Chem. Soc. 70 (10): 3426–3428. doi:10.1021/ja01190a060. PMID 18891879.
- ↑ Dumas, Aaron M.; Fillion, Eric (2009). "Meldrum's Acids and 5-Alkylidene Meldrum's Acids in Catalytic Carbon–Carbon Bond-Forming Processes". Acc. Chem. Res. 43 (3): 440–454. doi:10.1021/ar900229z.
- ↑ Lipson, Victoria V.; Gorobets, Nikolay Yu. (2009). "One hundred years of Meldrum's acid: Advances in the synthesis of pyridine and pyrimidine derivatives". Molec. Divers. 13 (4): 399–419. doi:10.1007/s11030-009-9136-x. PMID 19381852.
- ↑ Nakamura, Satoshi; Hirao, Hajime; Ohwada, Tomohiko (2004). "Rationale for the Acidity of Meldrum's Acid. Consistent Relation of C−H Acidities to the Properties of Localized Reactive Orbital". J. Org. Chem. 69 (13): 4309–4316. doi:10.1021/jo049456f. PMID 15202884.
- ↑ Oikawa, Yuji; Sugano, Kiyoshi; Yonemitsu, Osamu (1978). "Meldrum's acid in organic synthesis. 2. A general and versatile synthesis of β-keto esters". J. Org. Chem. 43 (10): 2087–2088. doi:10.1021/jo00404a066.
Further reading
- McNab, Hamish (1978). "Meldrum's Acid". Chem. Soc. Rev. 7 (3): 345–358. doi:10.1039/CS9780700345.
- Bonifácio, Vasco D. B. (2004). "Meldrum's Acid" (PDF). Synlett. 2004 (9): 1649–1650. doi:10.1055/s-2004-829539. Archived from the original (PDF) on September 27, 2007.
- Gerencsér, János; Dormán, György; Darvas, Ferenc (2006). "Meldrum's Acid in Multicomponent Reactions: Applications to Combinatorial and Diversity-Oriented Synthesis". 25 (5–6): 439–448. doi:10.1002/qsar.200540212.
- Ivanov, Andrey S. (2008). "Meldrum's acid and related compounds in the synthesis of natural products and analogs". Chem. Soc. Rev. 37 (4): 789–811. doi:10.1039/B716020H.
- Kidd, Hamish (October 29, 2008). "Meldrum's Acid". Chemistry World: 35–36.