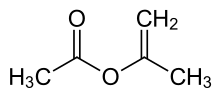
Isopropenyl acetate
 | |
| Names | |
|---|---|
| Other names
1-Methylvinyl acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number | 203-562-7 |
PubChem CID |
|
| |
| |
| Properties | |
| C5H8O2 | |
| Molar mass | 100.12 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9090 g/cm3 (20 ºC) |
| Melting point | −92.9 °C (−135.2 °F; 180.2 K) |
| Boiling point | 97 °C (207 °F; 370 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isopropenyl acetate is an organic compound, which is the acetate ester of the enol tautomer of acetone. This colorless liquid is significant commercially as the principal precursor to acetylacetone. In organic synthesis, it is used to prepare enol acetates of ketones and acetonides from diols.[1]
Preparation and reactions
Isopropenyl acetate is prepared by treating acetone with ketene.[2] Upon heating over a metal surface, isopropenyl acetate rearranges to acetylacetone.[3]
- CH2(CH3)COC(O)Me → MeC(O)CH2C(O)Me
References
- ↑ Michael A. Walters, Melissa D. Lee (2001). "Isopropenyl Acetate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ri077.
- ↑ Raimund Miller, Claudio Abaecherli, Adel Said, Barry Jackson "Ketenes" in Ullmann's Encyclopedia of Industrial Chemistry, 2001, Wiley-VCH, Weinheim. doi:10.1002/14356007.a15_063
- ↑ Siegel, Hardo; Eggersdorfer, Manfred (2002). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.