Locant
In organic chemistry, a locant is a figure to indicate the position of a functional group within a molecule.[1]
Example
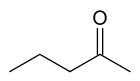
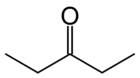
There are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located.
In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three or four. However, atoms two and four are exactly equivalent - which can be shown by turning the molecule around by 180 degrees.
The locant is the number of the carbon atom to which the oxygen atom is bonded. If the oxygen is bonded to the middle carbon, the locant is 3. If the oxygen is bonded to an atom on either side (adjacent to an end carbon), the locant is 2 or 4; given the choice here, where the carbons are exactly equivalent, the lower number is always chosen. So the locant is either 2 or 3 in this molecule.
The locant is incorporated into the name of the molecule to remove ambiguity. Thus the molecule is named either pentan-2-one or pentan-3-one, depending on the position of the oxygen atom.
Any side chains can be present in the place of oxygen and it can defined as simply the number on the carbon to which any thing other than a hydrogen is attached.