Lithium-ion capacitor

| Specific energy | 11–14 Wh/kg |
|---|---|
| Energy density | 19–25 Wh/L |
| Specific power | 160–2800 W/kg |
| Charge/discharge efficiency | 95% |
| Self-discharge rate | < 5% per month (temperature dependent) |
| Cycle durability | >10,000 |
| Nominal cell voltage | 2.2–3.8 V |
A lithium-ion capacitor (LIC) is a hybrid type of capacitor classified as a type of supercapacitor. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared with other supercapacitors.
History
In 1981, Dr. Yamabe of Kyoto University, in collaboration with Dr. Yata of Kanebo Co., created a material known as PAS (polyacenic semiconductive) by pyrolyzing phenolic resin at 400–700 °C.[1] This amorphous carbonaceous material performs well as the electrode in high-energy-density rechargeable devices. Patents were filed in the early 1980s by Kanebo Co.,[2] and efforts to commercialize PAS capacitors and lithium-ion capacitors (LICs) began. The PAS capacitor was first used in 1986,[3] and the LIC capacitor in 1991.
Concept
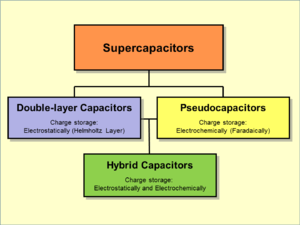
A lithium-ion capacitor is a hybrid electrochemical energy storage device which combines the intercalation mechanism of a lithium-ion battery anode with the double-layer mechanism of the cathode of an electric double-layer capacitor (EDLC). The packaged energy density of an LIC is approximately 20 Wh/kg, roughly four times higher than an EDLC and five times lower than a lithium-ion battery. The power density, however, has been shown to match that of EDLCs, as it is able to completely discharge in seconds.[4] At the negative electrode (cathode), for which activated carbon is often used, charges are stored in an electric double layer that develops at the interface between the electrode and the electrolyte.
The positive electrode (anode) was originally made from lithium titanate oxide, but is now more commonly made from graphitic carbon to maximize energy density. The graphitic electrode potential initially at -0.1 V versus SHE (standard hydrogen electrode) is lowered further to -2.8 V by intercalating lithium ions. This step is referred to as "doping" and often takes place in the device between the anode and a sacrificial lithium electrode. The pre-doping process is critical to the device functioning as it can significantly affect the development of the solid electrolyte interphase (SEI) layer. Doping the anode lowers the anode potential and leads to a higher output voltage of the capacitor. Typically, output voltages for LICs are in the range of 3.8–4.0 V but are limited to minimum allowed voltages of 1.8–2.2 V. If the voltage drops any lower than this lithium ions will deintercalate more rapidly than they can be restored during normal use. Like EDLCs, LIC voltages vary linearly adding to complications integrating them into systems which have power electronics that expect the more stable voltage of batteries. As a consequence, LICs have a high energy density, which varies with the square of the voltage. The capacitance of the anode is several orders of magnitude larger than that of the cathode. As a result, the change of the anode potential during charge and discharge is much smaller than the change in the cathode potential.
The electrolyte used in an LIC is a lithium-ion salt solution that can be combined with other organic components and is generally identical to that used in lithium-ion batteries.
A separator prevents direct electrical contact between anode and cathode.
Properties
Typical properties of an LIC are
- high capacitance compared to a capacitor, because of the large anode, though low capacity compared to a Li-ion cell
- high energy density compared to a capacitor(14 Wh/kg reported[5]), though low energy density compared to a Li-ion cell
- high power density
- high reliability
- operating temperatures ranging from −20 °C to 70 °C[6]
- low self-discharge (<5% voltage drop at 25 °C over three months)[6]
Comparison to other technologies

Batteries, EDLC and LICs each have different strengths and weaknesses, making them useful for different categories of applications. LICs have higher power densities than batteries, and are safer than lithium-ion batteries (LIBs), in which thermal runaway reactions may occur. Compared to the electric double-layer capacitor (EDLC), the LIC has a higher output voltage. Although they have similar power densities, the energy density of an LIC is much higher than those of the others.
The Ragone plot in figure 1 shows that LICs combine the high energy of LIBs with the high power density of EDLCs.
Cycle life performance of LICs is much better than batteries and is similar to EDLCs.
Applications
Lithium-ion capacitors are quite suitable for applications which require a high energy density, high power densities and excellent durability. Since they combine high energy density with high power density, there is no need for additional electrical storage devices in various kinds of applications, resulting in reduced costs.
Potential applications for lithium-ion capacitors are, for example, in the fields of wind power generation systems, uninterruptible power source systems (UPS), voltage sag compensation, photovoltaic power generation, energy recovery systems in industrial machinery, and transportation systems.
References
- ↑ Proceedings Annual Meeting of the Physical Society of Japan (Yokohama) 31p-K-1, 1982, March
- ↑ Japanese patent application No. 56-92626,1981
- ↑ International Conference on Science and Technology of Synthetic Metals 1986, Kyoto
- ↑ "Evaluation of lithium-ion capacitors assembled with pre-lithiated graphite anode and activated carbon cathode". Electrochimica Acta. 65: 280–287. 20 March 2012. doi:10.1016/j.electacta.2012.01.076. Retrieved 2014-01-14.
- ↑ "FDK To Begin Mass Production of High-Capacity Li-Ion Capacitors". 4 January 2009. Retrieved 2010-07-23.
- 1 2 "ULTIMO Li-ion hybrid capacitor Spec Sheet" (PDF).
External links
- Introducing JM Energy Lithium-Ion Capacitor, JM Energy
- Lithium-Ion Capacitor, JSR Micro