Lac repressor
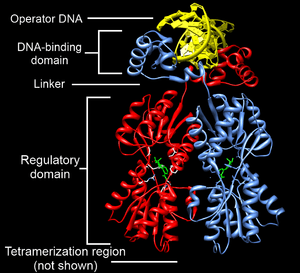
The lac repressor is a DNA-binding protein which inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria. These genes are repressed when lactose is not available to the cell, ensuring that the bacterium only invests energy in the production of machinery necessary for uptake and utilization of lactose when lactose is present. When lactose becomes available, it is converted into allolactose, which inhibits the lac repressor's DNA binding ability. Loss of DNA binding by the lac repressor is required for transcriptional activation of the operon.
Function
The lac repressor (LacI) operates by a helix-turn-helix motif in its DNA-binding domain, binding base-specifically to the major groove of the operator region of the lac operon, with base contacts also made by residues of symmetry-related alpha helices, the "hinge" helices, which bind deeply in the minor groove.[1] This bound repressor can reduce transcription of the Lac proteins by occluding the RNA polymerase binding site or by prompting DNA looping.[2] When lactose is present, allolactose binds to the lac repressor, causing an allosteric change in its shape. In its changed state, the lac repressor is unable to bind tightly to its cognate operator. Thus, the gene is mostly off in the absence of inducer and mostly on in the presence of inducer, although the degree of gene expression depends on the number of repressors in the cell and on the repressor's DNA-binding affinity.[3] Isopropyl β-D-1-thiogalactopyranoside (IPTG) is a commonly used allolactose mimic which can be used to induce transcription of genes being regulated by lac repressor.
Structure
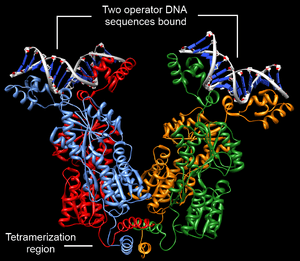
Structurally, the lac repressor protein is a homo-tetramer. The tetramer contains two DNA-binding subunits composed of two monomers each (sometimes called "dimeric lac repressor"). These subunits dimerize to form a tetramer capable of binding two operator sequences. Each monomer [4][5][6] consists of four distinct regions:
- an N-terminal DNA-binding domain (in which two LacI proteins bind a single operator site)
- a regulatory domain (sometimes called the core domain, which binds allolactose, an allosteric effector molecule)
- a linker that connects the DNA-binding domain with the core domain (sometimes called the hinge helix, which is important for allosteric communication[6])
- a C-terminal tetramerization region (which joins four monomers in an alpha-helix bundle)
DNA binding occurs via an N-terminal helix-turn-helix structural motif and is targeted to one of several operator DNA sequences (known as O1, O2 and O3). The O1 operator sequence slightly overlaps with the promoter, which increases the affinity of RNA polymerase for the promoter sequence such that it cannot enter elongation and remains in Abortive initiation. Additionally, because each tetramer contains two DNA-binding subunits, binding of multiple operator sequences by a single tetramer induces DNA looping.[7]
Search Kinetics and molecular models for binding and sliding
Questions remain about how DNA regulatory proteins, such as lacI, find their specific target sides efficiently. One theory is that lacI, and other Transcriptions Factor's (TF's), find their binding sides in a process of combined 3- and 1-dimensional diffusion. The 1D sliding reduces the dimensionality and facilitates the search process. This model has first been tested for lacI in vitro and later in living cells. LacI seems to slide over the O1 operator several times before it binds which is suggested to be a trade-off between fast searching on nonspecific and binding to specific sequences.[8] Sliding of the lac repressor on DNA is a molecular event that is impossible to observe even with today's imaging techniques, like fluorescent microscopy. Therefore digital molecular models have been proposed to represent the atoms of the protein and DNA interacting in 3D space. All-atom and coarse-grained[9] molecular dynamic simulations have both been used. In a all-atom simulations of Marklund et al. a helical sliding path on DNA has been studied using helical Umbrella Sampling. The resulting potential of mean force (PMF) suggests that the transcription factor encounters a barrier of 1 kBT for sliding and 12 kBT for dissociation. This can be transformed into a sliding distance of 8 bp on average before dissociating.[10]
The in vivo search model for the lac repressor includes intersegment transfer and hopping as well as crowding by other proteins which make the genome in E.coli cells less accessible for the repressor.[11] This macroscopic models can be studied with brownian simulations combined with fluorescent microscopy, which monitors the labelled lacI dimer in the cell.
Discovery
The lac repressor was first isolated by Walter Gilbert and Benno Müller-Hill in 1966.[12] They were able to show, in vitro, that the protein bound to DNA containing the lac operon, and released the DNA when IPTG was added. (IPTG is an allolactose analog.) They were also able to isolate the portion of DNA bound by the protein by using the enzyme deoxyribonuclease, which breaks down DNA. After treatment of the repressor-DNA complex, some DNA remained, suggesting that it had been masked by the repressor. This was later confirmed.
These experiments confirmed the mechanism of the lac operon, earlier proposed by Jacques Monod and Francois Jacob.
See also
References
- ↑ Schumacher MA, Choi KY, Zalkin H, Brennan RG (November 1994). "Crystal structure of LacI member, PurR, bound to DNA: minor groove binding by alpha helices". Science. 266 (5186): 763–70. doi:10.1126/science.7973627. PMID 7973627.
- ↑ Razo-Mejia M, Boedicker J, Jones D, DeLuna A, Kinney J, Phillips R (2014). "Comparison of the theoretical and real-world evolutionary potential of a genetic circuit". Physical Biology. 1 (2): 026005. doi:10.1088/1478-3975/11/2/026005. PMC 4051709. PMID 24685590.
- ↑ Razo-Mejia M, Barnes S, Belliveau N, Chure G, Einav T, Lewis M, Phillips R (2018). "Tuning Transcriptional Regulation through Signaling: A Predictive Theory of Allosteric Induction". Cell Systems. 6 (4): 456–469. doi:10.1016/j.cels.2018.02.004. PMC 5991102. PMID 29574055.
- ↑ Goodsell DS (2003). "Lac Repressor". RCSB Protein Data Bank. doi:10.2210/rcsb_pdb/mom_2003_3.
- ↑ Lewis M (June 2005). "The lac repressor". Comptes Rendus Biologies. 328 (6): 521–48. doi:10.1016/j.crvi.2005.04.004. PMID 15950160.
- 1 2 Swint-Kruse L, Matthews KS (April 2009). "Allostery in the LacI/GalR family: variations on a theme". Current Opinion in Microbiology. 12 (2): 129–37. doi:10.1016/j.mib.2009.01.009. PMC 2688824. PMID 19269243.
- ↑ Oehler S, Eismann ER, Krämer H, Müller-Hill B (April 1990). "The three operators of the lac operon cooperate in repression". The EMBO Journal. 9 (4): 973–9. PMC 551766. PMID 2182324.
- ↑ Hammar, Petter; Leroy, Prune; Mahmutovic, Anel; Marklund, Erik G.; Berg, Otto G.; Elf, Johan (2012-06-22). "The lac Repressor Displays Facilitated Diffusion in Living Cells". Science. 336 (6088): 1595–1598. doi:10.1126/science.1221648. ISSN 0036-8075. PMID 22723426.
- ↑ Bhattacherjee, Arnab; Krepel, Dana; Levy, Yaakov (2016-04-27). "Coarse-grained models for studying protein diffusion along DNA". Wiley Interdisciplinary Reviews: Computational Molecular Science. 6 (5): 515–531. doi:10.1002/wcms.1262. ISSN 1759-0876.
- ↑ Marklund, Erik G.; Mahmutovic, Anel; Berg, Otto G.; Hammar, Petter; Spoel, David van der; Fange, David; Elf, Johan (2013-12-03). "Transcription-factor binding and sliding on DNA studied using micro- and macroscopic models". Proceedings of the National Academy of Sciences. 110 (49): 19796–19801. doi:10.1073/pnas.1307905110. ISSN 0027-8424. PMC 3856812. PMID 24222688.
- ↑ Mahmutovic, Anel; Berg, Otto G.; Elf, Johan (2015-03-16). "What matters for lac repressor search in vivo—sliding, hopping, intersegment transfer, crowding on DNA or recognition?". Nucleic Acids Research. 43 (7): 3454–3464. doi:10.1093/nar/gkv207. ISSN 1362-4962. PMC 4402528. PMID 25779051.
- ↑ Gilbert W, Müller-Hill B (December 1966). "Isolation of the lac repressor". Proceedings of the National Academy of Sciences of the United States of America. 56 (6): 1891–8. doi:10.1073/pnas.56.6.1891. PMC 220206. PMID 16591435.
External links
- Lac+Repressors at the US National Library of Medicine Medical Subject Headings (MeSH)
- More information on the lac repressor molecule on protein database
- Lac Repressor in Proteopedia.