Karlsruhe Congress
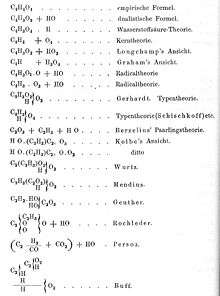
The Karlsruhe Congress was an international meeting of chemists held in Karlsruhe, Germany from 3 to 5 September 1860. It was the first international conference of chemistry worldwide.
The meeting
The Karlsruhe Congress was called so that European chemists could discuss matters of chemical nomenclature, notation, and atomic weights. The organization, invitation, and sponsorship of the conference were handled by August Kekulé, Adolphe Wurtz, and Karl Weltzien.[1] As an example of the problems facing the delegates, Kekulé's Lehrbuch der Organischen Chemie gave nineteen different formulas used by chemists for acetic acid, as shown in the figure on this page.[2][3]
The Karlsruhe meeting ended with no firm agreement on the vexing problem of atomic and molecular weights. However, on the meeting's last day reprints of Stanislao Cannizzaro's 1858 paper on atomic weights,[4] in which he utilized earlier work by Amedeo Avogadro, were distributed. Cannizzaro's efforts exerted a heavy and, in some cases, an almost immediate influence on the delegates. Lothar Meyer later wrote that on reading Cannizzaro's paper, "The scales seemed to fall from my eyes."[5][6]
An important long-term result of the Karlsruhe Congress was the adoption of the now-familiar atomic weights. Prior to the Karlsruhe meeting, and going back to Dalton's work in 1803, several systems of atomic weights were in use.[7] In one case, a value of 1 was adopted as the weight of hydrogen (the base unit), with 6 for carbon and 8 for oxygen. As long as there were uncertainties over atomic weights then the compositions of many compounds remained in doubt. Following the Karlsruhe meeting, values of about 1 for hydrogen, 12 for carbon, 16 for oxygen, and so forth were adopted. This was based on a recognition that certain elements, such as hydrogen, nitrogen, and oxygen, were composed of diatomic molecules and not individual atoms.
Ihde has argued[8] that the Karlsruhe meeting was the first international meeting of chemists and that it led to the eventual founding of the International Union of Pure and Applied Chemistry (IUPAC).
Attendance
According to Wurtz's list,[9] the congress was attended by the scientists listed below.
- Belgium:
- Germany:
- Berlin: Ad. Baeyer, G. Quinke;
- Bonn: H. Landolt;
- Breslau: Lothar Meyer;
- Kassel: Guckelberger,;
- Klausthal: Streng;
- Darmstadt: E. Winkler;
- Erlangen: v. Gorup-Besanez;
- Freiburg i. B.: v. Babo, Schneyder;
- Giessen: Boeckmann, H. Kopp, H. Will;
- Göttingen: F. Beilstein;
- Halle a. S.: W. Heintz;
- Hanover: Heeren;
- Heidelberg: Becker, O. Braun, R. Bunsen, L. Carius, E. Erlenmeyer, O. Mendius, Schiel;
- Jena: Lehmann, H. Ludwig;
- Karlsruhe: A. Klemm, R. Muller, J. Nessler, Petersen, K. Seubert, Weltzien;
- Leipzig: O. L. Erdmann, Hirzel, Knop, Kuhn;
- Mannheim: Gundelach, Schroeder;
- Marburg a. L.: R. Schmidt, Zwenger;
- Munich: Geiger;
- Nuremberg: v. Bibra;
- Offenbach: Grimm;
- Rappenau: Finck;
- Schönberg: R. Hoffmann;
- Speyer: Keller, Mühlhaüser;
- Stuttgart: v. Fehling, W. Hallwachs;
- Tübingen: Finckh, A. Naumann, A. Strecker;
- Wiesbaden: Kasselmann, R. Fresenius, C. Neubauer;
- Würzburg: Scherer, v. Schwarzenbach
- United Kingdom:
- Dublin: Apjohn A.;
- Edinburgh: Al. Crum Brown, Wanklyn, F. Guthrie;
- Glasgow: Anderson;
- London: B. F. Duppa, G. C. Foster, Gladstone, Müller, Noad, A. Normandy, Odling;
- Manchester: Roscoe;
- Oxford: Daubeny, G. Griffeth, F. Schickendantz;
- Woolwich: Abel
- France:
- Montpellier: A. Béchamp, A. Gautier, C. G. Reichauer;
- Mülhousen i. E.: Th. Schneider;
- Nancy: J. Nicklès;
- Paris: Boussingault, J-B. Dumas, C. Friedel, L. Grandeau, Le Canu, Persoz, Alf. Riche, P. Thénard, Verdét, C.-A. Wurtz;
- Strasbourg i. E.: Jacquemin, Oppermann, F. Schlagdenhaussen, P. Schützenberger;
- Tann: Ch. Kestner, Scheurer-Kestner
- Italy:
- Genoa: Cannizzaro;
- Pavia: Pavesi.
- Mexico: Posselt (Louis Posselt (1817–1880), brother of Christian Posselt)
- Austria:
- Innsbruck: Hlasiwetz;
- Lemberg: Pebal;
- Pesth: Th. Wertheim;
- Vienna: V. v. Lang, A. Lieben, Folwarezny, F. Schneider
- Portugal: Coïmbra: Mide Carvalho
- Russia:
- Kharkov: Sawitsch;
- St. Petersburg: Borodin, D. Mendeleev, L. Schischkoff, Zinin N.;
- Poland: Warsaw: T. Lesinski, J. Natanson;
- Sweden:
- Harpenden: J. H. Gilbert;
- Lund: Berlin, C. W. Blomstrand;
- Stockholm: Bahr
- Switzerland:
- Bern: C. Brunner, H. Schiff;
- Geneva: C. Marignac;
- Lausanne: Bischoff;
- Reichenau bei Chur: A. v. Planta;
- Zurich: J. Wislicenus
- Spain: Madrid: R. Torres Muñoz de Luna (He was wrongly referenced in the Wurtz's list as R. de Suna or Ramón de Luna)[10]
References
- ↑ Leicester, Henry M. (1956). The Historical Background of Chemistry. John Wiley and Sons. pp. 191&ndash, 192. ISBN 0-486-61053-5.
- ↑ Kekulé, A. (1861). Lehrbuch der Organischen Chemie … , vol. 1 (in German). Erlangen, (Germany): Ferdinand Enke. p. 58.
- ↑ The French chemist Auguste Laurent also listed many different contemporary representations of acetic acid (acide acétique) in his book: Laurent, Auguste (1854). Méthode de Chimie (in French). Paris, France: Mallet-Bachelier. pp. 27–28.
- ↑ See:
- Cannizzaro, Stanislao (1858). "Lettera del Prof. Stanislao Cannizzaro al Prof. S. de Luca; Sunto di un corso di filosofia chimica fatto nella Reale Università di Genova dal Professore S. Cannizzaro" [Letter from Prof. Stanislao Cannizzaro to Prof. S. de Luca; summary of a course of chemical philosophy taught at the Royal University of Genoa by Prof. S. Cannizzaro]. Il Nuovo Cimento (in Italian). 7: 321–366. Bibcode:1858NCim....7..321.. doi:10.1007/bf02827711.
- English translation: Cannizzaro, Stanislao (1911). Sketch of a Course of Chemical Philosophy. Edinburgh, Scotland: The Alembic Club.
- ↑ Moore, F. J. (1931). A History of Chemistry. McGraw-Hill. pp. 182&ndash, 184. ISBN 0-07-148855-3. (2nd edition)
- ↑ Cannizzaro, Stanislao (1891) with Arthur Miolati, trans., and Lothar Meyer, ed. Abriss eines Lehrganges der theoretischen Chemie [Outline of a Course of Theoretical Chemistry] (Leipzig, (Germany): Wilhelm Engelmann, 1891), p. 59. On p. 59, Lothmar Meyer wrote: "Nach Schluss der Versammlung vertheilte Freund Angelo Pavesi im Auftrage des Verfassers eine kleine ziemlich unscheinbare Schrift, den hier wiedergegeben "Sunto" etc. Cannizzaro's, der schon einige Jahre früher erschienen, aber wenig bekannt geworden war. Auch ich erhielt ein Exemplar, das ich einsteckte, um es unterwegs auf der Heimreise zu lesen. Ich las es wiederholt auch zu Hause und war erstaunt über die Klarheit, die das Schriftchen über die wichtigsten Streitpunkte verbreitete. Es fiel mir wie Schuppen von den Augen, die Zweifel schwanden, und das Gefühl ruhigster Sicherheit trat an ihre Stelle." (At the conclusion of the meeting, friend Angelo Pavesi, on behalf of the author, distributed a small, inconspicuous pamphlet, Cannizzaro's "Sunto" etc. [which is] reproduced here [Note: "Sunto" refers to: Stanislao Cannizzaro (1858) "Lettera del Prof. Stanislao Cannizzaro al Prof. S. de Luca; Sunto di un corso di filosofia chimica fatto nella Reale Università di Genova dal Professore S. Cannizzaro," Il Nuovo Cimento, 7 : 321–366.], which had appeared a few years earlier but has been little known. I too received a copy, which I pocketed to read on the way home. I also read it at home repeatedly and was amazed at the clarity that the pamphlet spread about the main issues. It was as if the scales fell from my eyes, the doubts faded, and the feeling of calmest assurance took its place.)
- ↑ An example of the confusion is provided by the table of atomic weights in the various prevailing systems, which appears in: Gehler, Johann Samuel Traugott (1840). Gmelin; Littrow; Muncke; Pfaff, eds. Johann Samuel Traugott Gehler's Physikalisches Wörterbuch, 9. Band, 3. Abtheilung [Johann Samuel Traugott Gehler's Physical Dictionary, vol. 9, part 3] (in German). Leipzig, (Germany): E.B. Schwickert. pp. 1909–1912. In the tables of pages 1911–1912, Column C presents the relative atomic weights of the known elements, assigning to hydrogen (Wasserstoff) an atomic weight of 1. Column D contains the same relative atomic weights as column C, except that oxygen (Sauerstoff) is assigned a relative atomic weight of 100. (Gehler says of columns C and D: "In den Columnen C und D finden sich die Atomgewichte, wie sie sich nach den so eben entwickelten Grundsätzen als die wahrscheinlichsten ergeben möchten, … " (In columns C and D are found the atomic weights, as they would result from the principles [that have been] developed just now as the most probable ones … ) But this system assigns to oxygen an atomic weight of 8 and to carbon (Kohlenstoff) an atomic weight of 6.) Column E presents the relative atomic weights according to Berzelius, who assigned to hydrogen atoms (das Atomgewicht des einfachen Wasserstoffatoms (the atomic weight of single hydrogen atoms)) a value of 0.5 and who found oxygen to have a value of 8.01 — about 16 times greater than that of the hydrogen atom, which is correct. Column F contains the same relative atomic weights as column E, except that oxygen is assigned a relative atomic weight of 100.
- ↑ Ihde, Aaron J. (1961). "The Karlsruhe Congress: A Centennial Retrospective". Journal of Chemical Education. 38 (2): 83&ndash, 86. Bibcode:1961JChEd..38...83I. doi:10.1021/ed038p83. (subscription required)
- ↑ See Charles-Adolphe Wurtz's report on the Karlsruhe Congress. Wurtz's list had "England" instead of "United Kingdom", and Warsaw was listed with Russia.
- ↑ Pellón, Inés; Bilbao-Goyoaga, Ana (2013). "The chemical atomic theory in Ramón Torres Muñoz de Luna's textbooks (1848-1885)". Circumscribere. 13: 46–65.
Further reading
- de Milt, Clara (1951). "The Congress at Karlsruhe". Journal of Chemical Education. 28 (8): 421&ndash, 425. Bibcode:1951JChEd..28..421D. doi:10.1021/ed028p421. (subscription required)
- Hartley, Harold (1966). "Stanislao Cannizzaro, F.R.S. (1826 – 1910) and the First International Chemical Conference at Karlsruhe". Notes and Records of the Royal Society of London. 21 (1): 56&ndash, 63. doi:10.1098/rsnr.1966.0006.
- Hudson, John (1992). The History of Chemistry. Chapman and Hall. pp. 122&ndash, 125. ISBN 0-12-007208-4.
- (Note the incorrect spelling of Weltzien's name.)
- Ihde, Aaron J. (1984). The Development of Modern Chemistry. Dover. pp. 228&ndash, 230. ISBN 0-486-64235-6.
- (Originally published in 1964.)
- Laing, Michael (November 1995). "The Karlsruhe Congress, 1860". Education in Chemistry. Vol. 32 no. 6. Royal Society of Chemistry. pp. 151&ndash, 153.
- Partington, J. R. (1951). A Short History of Chemistry. MacMillan and Company. pp. 256&ndash, 258. ISBN 0-486-65977-1.
- (Note the incorrect month given for the conference.)
- Nye, Mary Jo (1984). The Question of the Atom: From the Karlsruhe Congress to the First Solvay Conference, 1860-1911. Springer. ISBN 0-938228-07-2.