KAHA Ligation
The α-Ketoacid-Hydroxylamine (KAHA) Amide-Forming Ligation is a chemical reaction that is used to join two unprotected fragments in peptide synthesis.[1][2] It is an alternative to the Native Chemical Ligation (NCL).
Overview
An α-ketoacid at the C-terminus of one peptide fragment reacts with a hydroxylamine at the N-terminus of another to form a peptide bond (amide bond).
The reaction can happen in the presence of unprotected side chains, and it does not require any coupling reagents or catalysts. The only byproducts are water and CO2.

Type I KAHA Ligation (unmodified hydroxy group)
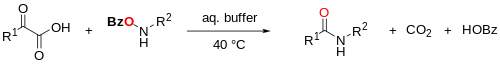
Type II KAHA Ligation (O-substituted hydroxylamine)
References
- ↑ Bode, Jeffrey W.; Fox, Ryan M.; Baucom, Kyle D. (2006). "Chemoselective Amide Ligations by Decarboxylative Condensations ofN-Alkylhydroxylamines and α-Ketoacids". Angewandte Chemie. 118 (8): 1270–1274. doi:10.1002/ange.200503991. ISSN 0044-8249.
- ↑ Pusterla, Ivano; Bode, Jeffrey W. (2012). "The Mechanism of the α-Ketoacid-Hydroxylamine Amide-Forming Ligation". Angewandte Chemie. 124 (2): 528–531. doi:10.1002/ange.201107198. ISSN 0044-8249.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.