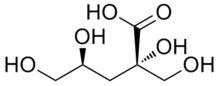
Isosaccharinic acid
 | |
| Names | |
|---|---|
| IUPAC name
(2S,4S)-2,4,5-Trihydroxy-2-(hydroxymethyl)pentanoic acid | |
| Other names
3-Deoxy-2-C-(hydroxymethyl)-D-erythro-pentonic acid; D-gluco-Isosaccharinic acid; Isosaccharinic acid; α-D-Glucoisosaccharinic acid; α-D-Isosaccharinic acid; α-Glucoisosaccharinic acid; α-Isosaccharinic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g·mol−1 |
| Melting point | 189 to 194 °C (372 to 381 °F; 462 to 467 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isosaccharinic acid (ISA) is a six-carbon sugar acid which is formed by the action of calcium hydroxide on lactose and other carbohydrates. It is of interest because it may form in intermediate-level nuclear waste stores when cellulose is degraded by the calcium hydroxide in cements such as Portland cement. The calcium salt of the alpha form of ISA is very crystalline and quite insoluble in cold water, but in hot water it is soluble.
ISA is thought to form by means of a series of reactions in which calcium ions acting as lewis acids catalyze two of the three steps. The first step is likely to be a rearrangement of the reducing sugar end of the cellulose (or lactose) into a keto sugar, the second step is likely to be a reaction similar to the base catylised dehydration which oftein occurs after an aldol reaction. In this second step an alkoxide (derived from a sugar) takes the role of the hydroxide leaving group, this second step is not likely to require the lewis acidity of the calcium. The final step is a benzilic acid rearrangement from a 1,2-diketone (1,5,6-trihydroxyhexane-2,3-dione) which is formed from the carbohydrate.
Under acidic conditions sugars tend to form furans such as furfural and 5-hydroxymethylfurfural by a series of dehydrations of the carbohydrate.
In acidic solutions the acid tends to form a 5-membered ring (lactone) by forming an ester between the carboxylic acid group and one of the alcohols. When treated with under anhydrous conditions with acetone, an acid and a dehydration agent two of the alcohol groups can be protected as a cyclic acetone acetal thus leaving behind only one alcohol,2 prolonged treatment with 2,2-dimethoxypropane forms a protected form of ISA where all four of the alcohol groups are protected as acetone acetals and the carboxylic acid is in the form of the methyl ester.3 These protected forms of ISA have been used as a starting material for chiral organic compounds anthracyclines.2
Relevance to United Kingdom Nuclear Waste Disposal Concept
The diastereomers of isosaccharinic acid have received particular attention in the literature due to its ability to complex a range of radionuclides, potentially affecting the migration of the radionuclides. ISAs are formed as a result of interactions between cellulosic materials present within the intermediate level waste inventory of the United Kingdoms nuclear legacy and alkalinity resulting from the use of cementitious materials in the construction of disposal concepts. Work by Greenfield et al., found that ISA and constituents formed in a cellulose degradation leachate were capable of forming soluble complexes with thorium, uranium (IV) and plutonium [6]. In the case of plutonium, ISA concentrations above 10−5 M were capable of increasing solubility above pH 12.0, where concentrations of 1-5 x 10−3 M were found to increase the solubility by an order of magnitude from 10−5 to 10−4 M. The work of Allard et al. [7] found that a concentration of ISA of 2 x 10−3 M could increase plutonium solubility by a factor of 2 x 105. In addition a range of studies on the complexation properties of α-isosaccharinic acid in alkaline solutions with various metals, including nickel, thorium, americium and europium have been conducted [8-12].
The work of Vercammen et al. [8] showed that although Ca(α-ISA)2 is sparingly soluble [13], both europium and thorium were capable of forming soluble complexes with ISA between pH 10.7 and 13.3, where a mixed metal complex was observed in the presence of thorium. Wieland also observed that α-ISA prevented the uptake of thorium by hardened cement pastes [11]. The works of Warwick et al. have also shown that ISA is capable of influencing the solubility both uranium and nickel through complexation [9,10]. Tits et al. [12] observed that in the absence of ISA, europium, americium and thorium will sorb to the calcite present within an ILW GDF. Should ISA concentrations within the facility exceed 10−5 mol L-1 (2x10−5 mol L−1 in the case of Th(IV)), it was reported that the sorption onto the calcite would be significantly affected such that the radionuclides studied would no longer be sorbed to the cement and instead be complexed with the ISA.
Most recently, work was carried out in order to determine the effect of cellulose degradation products on radionuclide solubility and sorption [14]. Cellulose degradation product leachates were first produced by contacting cellulose sources (wood, rad wipes or cotton wool) with calcium hydroxide (pH 12.7) under anaerobic conditions. Analysis of the leachates across 103 days suggested that the primary product of the degradation was ISA, although a range of other organic compounds were formed and varied across cellulose source. In these experiments both ISA and X-ISA were able to increase the solubility of europium at pH 12, where in experiments with thorium ISA had a more profound effect on thorium solubility than that of X-ISA, where little effect was observed.
Microbial Activity Within a Nuclear Waste Disposal Concept
ISA also represents a major carbon source within a geological disposal facility since it comprises >70% of cellulose degradation products as a result of alkaline hydrolysis . The high pH associated with such a facility means that microbial activity may or may not occur within the alkaline disturbed zone depending on the local microbial consortia intruding upon or surrounding such a facility post closure. Initial studies have shown that both alpha and beta forms of ISA are readily available for microbial action under the anaerobic conditions expected within the far field of a disposal facility or within ungrouted waste packages [15]. Since the evolution of pH within a facility is expected to fall from 13.5 to 10-12.5 over tens of thousands of years, the ability of micro-organisms to adapt to these alkaline pH values has also been investigated. Mesophilic consortia have been shown to adapt to a pH of 10 within a number of weeks, ISA degradation ceased above pH 11.0 [16]. Microbial consortia from hyperalkaline environments in which exposure to >pH 11.0 has occurred for over a century have also been exposed to ISA's generated from the alkaline hydrolysis of organic matter in situ. This consortia was readily capable of degrading ISA's [17] , with the same consortia was also capable of existing as polymicrobial flocculates, which has shown to be capable of survival up to pH 12.5 [18]. As a result, the impact of microbial activity within a GDF is expected to be through the degradation of ISA's and production of gas, which may impact on facility pressurisation but also through the generation of 14C bearing gases [19].
References
- ↑ Whistler, Roy L.; Richards, G. N. (1958). "Uronic acid fragments from slash pine (Pinus elliotti) and their behavior in alkaline solution". Journal of the American Chemical Society. 80 (18): 4888–4891. doi:10.1021/ja01551a031.
2. Florent, J. C.; Ughetto-Monfrin, J.; Monneret, C., Journal of Organic Chemistry, 1987, volume 52, issue 6, pages 1051-1056.
3. Florent, Jean-Claude; Genot, Agnes; Monneret, Claude, Tetrahedron letters, 1985, vol 26, issue 43, pages 5295 - 5298.
4. Whistler; Be Miller, Journal of the American Chemical Society, 1960, volume 82, page 3705.
5. P N Humphreys, A Laws, J Dawson (2010) A Review of Cellulose Degradation and the Fate of Degradation Products Under Repository Conditions. SERCO / TAS / 002274 / 001. Serco Contractors Report for the Nuclear Decommissioning authority, UK.
6. Greenfield B, Hurdus M, Spindler M, Thomason H (1997) The effects of the products from the anaerobic degradation of cellulose on the solubility and sorption of radioelements in the near field NSS/R375 AEA Technology plc, Harwell, Didcot, Oxfordshire, UK.
7. Allard S, Ekberg C (2006) Complexing Properties of α-Isosaccharinate: Stability Constants, Enthalpies and Entropies of Th-complexation with Uncertainty Analysis. Journal of Solution Chemistry 35 8: 1173-1186.
8. Vercammen K, Glaus M, Van Loon LR (2001) Complexation of Th (IV) and Eu (III) by α-isosaccharinic acid under alkaline conditions. Radiochimica Acta 89 6/2001: 393.
9. Warwick P, Evans N, Hall T, Vines S (2003) Complexation of Ni(II) by α-isosaccharinic acid and gluconic acid from pH 7 to pH 13. Radiochimica Acta 91 4-2003: 233-240.
10. Warwick P, Evans N, Hall T, Vines S (2004) Stability constants of uranium (IV)-α-isosaccharinic acid and gluconic acid complexes. Radiochimica Acta/International journal for chemical aspects of nuclear science and technology 92 12/2004: 897-902.
11. Wieland E, Tits J, Dobler J, Spieler P (2002) The effect of α-isosaccharinic acid on the stability of and Th (IV) uptake by hardened cement paste. Radiochimica Acta 90 9-11/2002: 683-688.
12. Tits J, Wieland E, Bradbury M (2005) The effect of isosaccharinic acid and gluconic acid on the retention of Eu (III), Am (III) and Th (IV) by calcite. Applied Geochemistry 20 11: 2082-2096.
13. Rai D, Rao L, Xia Y (1998) Solubility of crystalline calcium isosaccharinate. Journal of Solution Chemistry 27 12: 1109-1122.
14. Randall M, Rigby B, Thomson O, Trivedi D (2013) Assessment of the effects of cellulose degradation products on the behaviour of europium and thorium 12239 Part A Issue 4 National Nuclear Laboratory, Chadwick House, Warington, UK.
15. Rout SP, Radford J, Laws AP, Sweeney F, Elmekawy A, et al. (2014) Biodegradation of the Alkaline Cellulose Degradation Products Generated during Radioactive Waste Disposal. PLoS ONE 9 9: e107433.
16. Rout SP, Charles CJ, Doulgeris C, McCarthy AJ, Rooks DJ, Loughnane JP, et al. (2015) Anoxic Biodegradation of Isosaccharinic Acids at Alkaline pH by Natural Microbial Communities. PLoS ONE 10(9): e0137682. doi:10.1371/journal. pone.0137682
17. Rout SP, Charles CJ, Garratt EJ, Laws AP, Gunn J, Humphreys PN (2015) Evidence of the Generation of Isosaccharinic Acids and Their Subsequent Degradation by Local Microbial Consortia within Hyper-Alkaline Contaminated Soils, with Relevance to Intermediate Level Radioactive Waste Disposal. PLoS ONE 10(3): e0119164. doi:10.1371/journal.pone.0119164
18. Charles CJ, Rout SP, Garratt EJ, Patel K, Laws AP, et al. (2015) The enrichment of an alkaliphilic biofilm consortia capable of the anaerobic degradation of isosaccharinic acid from cellulosic materials incubated within an anthropogenic, hyperalkaline environment. FEMS Microbial Ecology Aug;91(8):fiv085. doi: 10.1093/femsec/fiv085. Epub 2015 Jul 20.
19. Doulgeris C, Humphreys PN, Rout S (2015) An approach to modelling the impact of 14C release from reactor graphite in a geological disposal facility. Mineralogical Magazine 79 (6) DOI: 10.1180/minmag.2015.079.6.24