Interfacial polymerization
Interfacial polymerization is a type of step-growth polymerization in which polymerization occurs at an interface between an aqueous solution containing one monomer and an organic solution containing a second monomer.[1][2] The process was invented by Chemist (later Laboratory Director) Emerson Wittbecker in 1959, and he used the term “interfacial polycondensation” when studied this method.[3] The most common polymer made by this method is polyamide, where diamine and diacid chloride react to form polyamide and hydrogen chloride.[4]
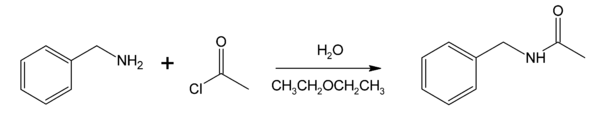
Mechanism
Most interfacial polymerization reactions utilize the Schotten-Baumann reaction mechanism under standard conditions, in which a diacid chloride in the organic phase reacts with a monomer containing hydrogen atoms available for reaction. This approach can be used to manufacture polyamides, polyesters, polyurethanes, polysulfonamides and polycarbonates. Because the two solutions used in this reaction are immiscible and the rate of reaction is high, this reaction mechanism tends to produce a small number of long polymer chains of high molecular weight.[5] As the polymer precipitates, it can be withdrawn continuously.
See also
References
- ↑ Odian, George (2004). Principles of Polymerization (4th ed.). Wiley-Interscience. pp. 90–92. ISBN 0471274003.
- ↑ Alger, Mark (1996). Polymer science dictionary (2nd ed.). London: Chapman & Hall. ISBN 0412608707.
- ↑ Wittbecker, Emerson L.; Morgan, Paul (1959). "Interfacial Polycondensation,". Journal of Polymer Science. 40 (137): 289. Bibcode:1959JPoSc..40..289W. doi:10.1002/pol.1959.1204013701.
- ↑ Chu, edited by Ravindra Pogaku, Awang Bono, Christopher (2013). Developments in Sustainable Chemical and Bioprocess Technology (Aufl. 2013 ed.). Boston, MA: Springer US. p. 112. ISBN 9781461462088.
- ↑ MacRitchie, F. (1969). "Mechanism of interfacial polymerization". Transactions of the Faraday Society. 65: 2503. doi:10.1039/TF9696502503.