Industrial dye degradation
Synthetic dyes are found in a wide range of products such as clothes, leather accessories, and furniture. These dyes are commonly used every day. However, a side effect of their widespread use is that up to 12% of these dyes are wasted during the dying process and about 20% of this wastage enters the environment (mainly into water supply).[1]

Dye degradation is a process in which the large dye molecules are broken down chemically into smaller molecules. The resulting products are water, carbon dioxide, and mineral byproducts that give the original dye its color. During the dyeing process, not all of the dye molecules are used. The water waste that the industry releases contains a percentage of these dye molecules. Dye molecules persist in the environment because many of them are not reactive towards light, acids, bases and oxygen. The color of the material becomes permanent.
Photocatalysis
Heterogeneous photocataylsis is a widely accepted technique of choice for environmental purification.[2] The standard experimental set up for dye degradation photocatalysis is by using a UV lamp to provide energy for the creation of oxidizing radicals. Photocatalysis is the addition of light to a semiconductor oxide/sulphide that results in electrons moving from the valence band to the conduction band. The electron-hole pairs formed will react with oxygen and water molecules to create superoxide anions and hydroxide radicals that have increased oxidizing and reducing abilities to be used on numerous industrial dye compounds.
Methods
6,13-Pentacenequinone/ Titanium Dioxide
Titanium dioxide (TiO2) is biologically stable, non-toxic, and cheap, which makes it a very common semiconductor for dye degradation. Due to its large band gap, some alterations can be made to improve its photocatalytic abilities such as the synthesis of 6,13-pentacenequinone/ TiO2. Titanium dioxide in conjunction with ultraviolet light can be utilized for the decolorization and detoxification of diluted colored water waste such as Alizarin, azo dyes, methyl red, methylene blue, etc.[3] Reduced graphene oxide-TiO2 can act as photocatalyst for the degradation of methyl orange, azo-dye, and pharmaceutical water waste.[4]
CuS
3-D structures of copper sulfide (CuS) is favored for methylene blue degradation because it is nontoxic, inexpensive, and stable under ambient conditions. It has efficient catalytic ability because of its high surface area to volume ratio allowing for better contact between the reactants and CuS.[5]
Graphitic Carbon Nitride
Hierarchically porous graphitic carbon nitride (hp-g-CN) had a 90% photodegradation of methyl orange, which is an improvement over other commercial photocatalysts.[6] This is due to a higher surface area for an increased absorption capacity and porous features that allow for an increased diffusion of methyl orange.
Fenton Process
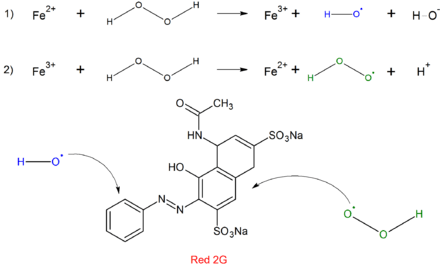
The Fenton process utilizes iron catalysts with H2O2 to create powerful, oxidizing hydroxides for the degradation of organic pollutants such as sewage and sludge as well as dyes. To enhance the catalytic abilities, a combination of Fe2+ cations, ultraviolet light, and hydrogen peroxide can be used and has shown greater removals of dye solutions.[7]
Biomass
Biomass degradation refers to the utilization of microorganisms such as bacteria and fungi to produce enzymes that can interact with molecules of dyes. Laccases are proteins that are produced by Lentinus sp; its active site contains a group of polyphenol oxidases incorporated with four copper ions. These can form hydrogen bonds with synthetic dyes. The efficiency of this enzyme is proportional to the number of hydrogen bonds that form between the enzyme and dyes.[8] Microorganisms are easy to manipulate, but the efficiency is highly dependent on the pH, ionic strength, and temperature. This will be varied with different effluents.[9] Effluents can be first processed by a strain of yeast Candida tropicalis JKS2 then post-treated by photocatalytic processes to degrade the aromatic rings so a cost-effective outcome can be achieved.[10] Immobilized fungal cells are more resistant to environmental stress and cells can be used repeatedly.[11]
Hazards
Many dyes, specifically in the textile industry such as methylene blue or methyl red, are released into ecosystems through water waste.[12] Many of these dyes can be carcinogenic and can come into contact with humans. As a result, newer treatments of the water waste are still in development.
References
- ↑ Rauf, M.A.; Ashraf S.S. Fundamental Principles and Application of Heterogeneous Photocatalytic Degradation of Dyes in Solution. Chemical Engineering Journal 2009, 151, 10-18.
- ↑ Pandit, V.K.; Arbuj, S.S.; Pandit, Y.B.; Naik, S.D.; Rane, S.B.; Mulik, U.P.; Gosavic, S.W.; Kale, B.B. Solar Light driven Dye Degradation using novel Organo–Inorganic (6,13-Pentacenequinone/TiO2) Nanocomposite”. RSC Adv. 2015, 5, 10326-10331.
- ↑ Lachheba, H.; Puzenata, E.; Houasb, A.; Ksibib, M.; Elalouib, E.; Guillarda, C.; Herrmanna, J. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated Titania. Applied Catalysis B: Environmental 2002, 39 (1), 75-90.
- ↑ Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Applied Catalysis B: Environmental 2012, 19 (9), 3676-3687.
- ↑ Shu, Q.W.; Lan, J.; Gao, M.X.; Wangb, J.; Huang, C.Z. Controlled synthesis of CuS caved superstructures and their application to the catalysis of organic dye degradation in the absence of light. CrystEngComm 2015, 17, 1374-1380.
- ↑ Gu, S.; Xieb, J.; Li, C.M. Hierarchically porous graphitic carbon nitride: large-scale facile synthesis and its application toward photocatalytic dye degradation. RSC Adv. 2014, 4, 59436-59439.
- ↑ Xu, X.; Li, H.; Wang, W.; Gu, J. Degradation of dyes in aqueous solutions by the Fenton process. Chemosphere 2004, 57, 595-600.
- ↑ Hsu, C.A.; Wen, T.N.; Su, Y.C.; Jiang, Z.B.; Chen, C.W.; Shyur, L.F. Biological Degradation of Anthroquinone and Azo Dyes by a Novel Laccase from Lentinus sp. Environmental Science & Technology 2012, 46 (9), 5109-5117.
- ↑ Prachi, K.; Anushree, M. Fungal dye decolourization: recent advances and future potential. Environment International 2009, 35 (1), 127-41.
- ↑ Jafari, N.; Kasra-Kermanshahi, R.; Soud, M.R.; Mahvi, A.H.; Gharavi, S. Degradation of a textile reactive azo dye by a combined biological-photocatalytic process: Candida tropicalis Jks2 -Tio2/Uv. Iranian Journal of Environmental Health Science & Engineering 2012, 9 (33), 1-7.
- ↑ Couto, S.R. Dye removal by Immobilised fungi. Biotechnology Advances 2009, 27 (3), 227-235.
- ↑ Huang, C.; Y. Huang; H. Cheng; Y. Huang. Kinetic Study of an Immobilized Iron Oxide for Catalytic Degradation of Azo Dye Reactive Black B with Catalytic Decomposition of Hydrogen Peroxide. Catalysis Communications 2009, 10 (5), 561-566.