Imidazolidinone
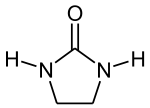
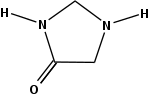
Imidazolidinones are a class of 5-membered ring heterocycles structurally related to imidazole. Imidazolidinones feature a saturated C3N2 backbones, except for the presence of a urea or amide functional group in the 2 or 4 positions.
2-Imidazolidinones
The 2-imidazolidinones are cyclic ureas. 1,3-Dimethyl-2-imidazolidinone is a polar solvent and Lewis base. Drugs featuring this ring system include emicerfont, imidapril, and azlocillin. Dimethylol ethylene urea is the reagent used in permanent press clothing.
4-Imidazolidinones
The 4-imidazolidinones are cyclic amides. Several have been investigated as organocatalysts.[1] Drugs featuring this ring include hetacillin, NNC 63-0532, spiperone, and spiroxatrine.
Imidazolones
Imidazolones (also called imidazolinones) are oxo derivatives of imidazoline (dihydroimidazoles). Examples include imidazol-4-one-5-propionic acid, a product of the catabolism of histidine, and imazaquin, a member of the imidazolinone class of herbicide.
References
- ↑ Barbara J. Morgan; Marisa C. Kozlowski; Aiguo Song; Wei Wang (2016). "(5S)-2,2,3-Trimethyl-5-(phenylmethyl)-4-imidazolidinone". eEROS. doi:10.1002/047084289X.rn00807.pub2.