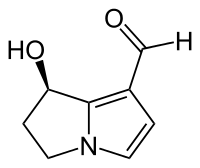
Hydroxydanaidal
 | |
 | |
| Names | |
|---|---|
| IUPAC name
7-Hydroxy-6,7-dihydro-5H-pyrrolizin-1-carboxaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| |
| Properties | |
| C8H9NO2 | |
| Molar mass | 151.17 g·mol−1 |
| Appearance | Solid |
| Melting point | 55 °C (131 °F; 328 K) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydroxydanaidal is an insect pheromone synthesized by some species of moth from pyrrolizidine alkaloids found in their diet.
Use by insects
The caterpillars of several kinds of arctiid moth ingest pyrrolizidine alkaloids—substances which plants produce to deter herbivores—and use them as protection against insectivores. The larva converts the alkaloid into an N-oxide for storage; on metamorphosis, the alkaloid is retained and used by the adult. This was discovered after the observation that Utetheisa ornatrix moths caught in spider-webs were cut loose by the spider.
Many insects that carry pyrrolizidine alkaloids are brightly-colored to signal their toxicity.
Male tiger moths convert the alkaloid through various intermediate stages[2] into the pheromone hydroxydanaidal;[3] female moth prefer males with more pheromone, since the alkaloid will be transferred by the male into her eggs and will protect them from predation.
References
- ↑ RÖMPP Lexikon Naturstoffe. Stuttgart: Verlag Thieme. 1997. p. 170. ISBN 978-3137499015.
- ↑ S Schulz; W Francke; M Boppré; T Eisner; J Meinwald (Jul 15, 1993). "Insect pheromone biosynthesis: stereochemical pathway of hydroxydanaidal production from alkaloidal precursors in Creatonotos transiens (Lepidoptera, Arctiidae)". PNAS. 90 (14): 6834–6838. Bibcode:1993PNAS...90.6834S. doi:10.1073/pnas.90.14.6834. PMC 47027. PMID 11607415.
- ↑ J A Edgar, M Boppré, E Kaufmann; Boppré; Kaufmann (2007). "Insect-synthesised Retronecine Ester Alkaloids: Precursors of the Common Arctiine (Lepidoptera) Pheromone Hydroxydanaidal". J Chem Ecol. 33 (12): 2266–2280. doi:10.1007/s10886-007-9378-y. PMID 18030534.