HIST1H2AK
Histone H2A type 1 is a protein that in humans is encoded by the HIST1H2AK gene.[5][6][7]
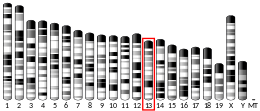
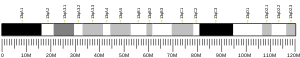
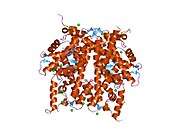
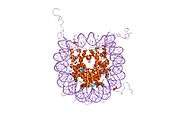
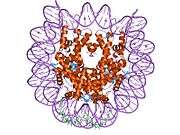
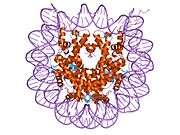
Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Two molecules of each of the four core histones (H2A, H2B, H3, and H4) form an octamer, around which approximately 146 bp of DNA is wrapped in repeating units, called nucleosomes. The linker histone, H1, interacts with linker DNA between nucleosomes and functions in the compaction of chromatin into higher order structures. This gene is intronless and encodes a member of the histone H2A family. Transcripts from this gene lack polyA tails but instead contain a palindromic termination element. This gene is found in the small histone gene cluster on chromosome 6p22-p21.3.[7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000275221 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000069270 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Albig W, Doenecke D (Feb 1998). "The human histone gene cluster at the D6S105 locus". Hum Genet. 101 (3): 284–94. doi:10.1007/s004390050630. PMID 9439656.
- ↑ Marzluff WF, Gongidi P, Woods KR, Jin J, Maltais LJ (Oct 2002). "The human and mouse replication-dependent histone genes". Genomics. 80 (5): 487–98. doi:10.1016/S0888-7543(02)96850-3. PMID 12408966.
- 1 2 "Entrez Gene: HIST1H2AK histone cluster 1, H2ak".
Further reading
- El Kharroubi A, Piras G, Zensen R, Martin MA (1998). "Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter". Mol. Cell. Biol. 18 (5): 2535–44. doi:10.1128/mcb.18.5.2535. PMC 110633. PMID 9566873.
- Albig W, Trappe R, Kardalinou E, et al. (1999). "The human H2A and H2B histone gene complement". Biol. Chem. 380 (1): 7–18. doi:10.1515/BC.1999.002. PMID 10064132.
- Deng L, de la Fuente C, Fu P, et al. (2001). "Acetylation of HIV-1 Tat by CBP/P300 increases transcription of integrated HIV-1 genome and enhances binding to core histones". Virology. 277 (2): 278–95. doi:10.1006/viro.2000.0593. PMID 11080476.
- Deng L, Wang D, de la Fuente C, et al. (2001). "Enhancement of the p300 HAT activity by HIV-1 Tat on chromatin DNA". Virology. 289 (2): 312–26. doi:10.1006/viro.2001.1129. PMID 11689053.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Mungall AJ, Palmer SA, Sims SK, et al. (2003). "The DNA sequence and analysis of human chromosome 6". Nature. 425 (6960): 805–11. doi:10.1038/nature02055. PMID 14574404.
- Lusic M, Marcello A, Cereseto A, Giacca M (2004). "Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter". EMBO J. 22 (24): 6550–61. doi:10.1093/emboj/cdg631. PMC 291826. PMID 14657027.
- Zhang Y, Griffin K, Mondal N, Parvin JD (2004). "Phosphorylation of histone H2A inhibits transcription on chromatin templates". J. Biol. Chem. 279 (21): 21866–72. doi:10.1074/jbc.M400099200. PMID 15010469.
- Aihara H, Nakagawa T, Yasui K, et al. (2004). "Nucleosomal histone kinase-1 phosphorylates H2A Thr 119 during mitosis in the early Drosophila embryo". Genes Dev. 18 (8): 877–88. doi:10.1101/gad.1184604. PMC 395847. PMID 15078818.
- Wang H, Wang L, Erdjument-Bromage H, et al. (2004). "Role of histone H2A ubiquitination in Polycomb silencing". Nature. 431 (7010): 873–8. doi:10.1038/nature02985. PMID 15386022.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Andersen JS, Lam YW, Leung AK, et al. (2005). "Nucleolar proteome dynamics". Nature. 433 (7021): 77–83. doi:10.1038/nature03207. PMID 15635413.
- Hagiwara T, Hidaka Y, Yamada M (2005). "Deimination of histone H2A and H4 at arginine 3 in HL-60 granulocytes". Biochemistry. 44 (15): 5827–34. doi:10.1021/bi047505c. PMID 15823041.
- Bonenfant D, Coulot M, Towbin H, et al. (2006). "Characterization of histone H2A and H2B variants and their post-translational modifications by mass spectrometry". Mol. Cell. Proteomics. 5 (3): 541–52. doi:10.1074/mcp.M500288-MCP200. PMID 16319397.
- Cao R, Tsukada Y, Zhang Y (2006). "Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing". Mol. Cell. 20 (6): 845–54. doi:10.1016/j.molcel.2005.12.002. PMID 16359901.
- Boyne MT, Pesavento JJ, Mizzen CA, Kelleher NL (2006). "Precise characterization of human histones in the H2A gene family by top down mass spectrometry". J. Proteome Res. 5 (2): 248–53. doi:10.1021/pr050269n. PMID 16457589.
- Bergink S, Salomons FA, Hoogstraten D, et al. (2006). "DNA damage triggers nucleotide excision repair-dependent monoubiquitylation of histone H2A". Genes Dev. 20 (10): 1343–52. doi:10.1101/gad.373706. PMC 1472908. PMID 16702407.