GALNT2
Polypeptide N-acetylgalactosaminyltransferase 2 is an enzyme that in humans is encoded by the GALNT2 gene.[5][6][7]
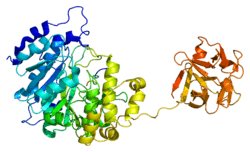
This gene encodes polypeptide N-acetylgalactosaminyltransferase 2, a member of the GalNAc-transferases family. This family transfers an N-acetyl galactosamine to the hydroxyl group of a serine or threonine residue in the first step of O-linked oligosaccharide biosynthesis. The localization site of this particular enzyme is preponderantly the trans-Golgi.[8] Individual GalNAc-transferases have distinct activities, and initiation of O-glycosylation in a cell is regulated by a repertoire of GalNAc-transferases.[7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000143641 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000089704 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Bennett EP, Weghuis DO, Merkx G, van Kessel AG, Eiberg H, Clausen H (Jul 1998). "Genomic organization and chromosomal localization of three members of the UDP-N-acetylgalactosamine: polypeptide N-acetylgalactosaminyltransferase family". Glycobiology. 8 (6): 547–55. doi:10.1093/glycob/8.6.547. PMID 9592121.
- ↑ White T, Bennett EP, Takio K, Sorensen T, Bonding N, Clausen H (Dec 1995). "Purification and cDNA cloning of a human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase". J Biol Chem. 270 (41): 24156–65. doi:10.1074/jbc.270.41.24156. PMID 7592619.
- 1 2 "Entrez Gene: GALNT2 UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2 (GalNAc-T2)".
- ↑ "African Swine Fever Virus Causes Microtubule-Dependent Dispersal of trans-Golgi Network" (PDF).
Further reading
- Bennett EP, Hassan H, Clausen H (1996). "cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase, GalNAc-t3". J. Biol. Chem. 271 (29): 17006–12. doi:10.1074/jbc.271.29.17006. PMID 8663203.
- Wandall HH, Hassan H, Mirgorodskaya E, et al. (1997). "Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, -T2, and -T3". J. Biol. Chem. 272 (38): 23503–14. doi:10.1074/jbc.272.38.23503. PMID 9295285.
- Müller S, Goletz S, Packer N, et al. (1997). "Localization of O-glycosylation sites on glycopeptide fragments from lactation-associated MUC1. All putative sites within the tandem repeat are glycosylation targets in vivo". J. Biol. Chem. 272 (40): 24780–93. doi:10.1074/jbc.272.40.24780. PMID 9312074.
- Röttger S, White J, Wandall HH, et al. (1998). "Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus". J. Cell Sci. 111 (1): 45–60. PMID 9394011.
- Mattu TS, Pleass RJ, Willis AC, et al. (1998). "The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fc alpha receptor interactions". J. Biol. Chem. 273 (4): 2260–72. doi:10.1074/jbc.273.4.2260. PMID 9442070.
- Iwasaki H, Zhang Y, Tachibana K, et al. (2003). "Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2". J. Biol. Chem. 278 (8): 5613–21. doi:10.1074/jbc.M211097200. PMID 12438318.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Marcos NT, Cruz A, Silva F, et al. (2003). "Polypeptide GalNAc-transferases, ST6GalNAc-transferase I, and ST3Gal-transferase I expression in gastric carcinoma cell lines". J. Histochem. Cytochem. 51 (6): 761–71. doi:10.1177/002215540305100607. PMID 12754287.
- Kinarsky L, Suryanarayanan G, Prakash O, et al. (2004). "Conformational studies on the MUC1 tandem repeat glycopeptides: implication for the enzymatic O-glycosylation of the mucin protein core". Glycobiology. 13 (12): 929–39. doi:10.1093/glycob/cwg109. PMID 12925576.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Gregory SG, Barlow KF, McLay KE, et al. (2006). "The DNA sequence and biological annotation of human chromosome 1". Nature. 441 (7091): 315–21. doi:10.1038/nature04727. PMID 16710414.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.