Freshwater acidification
Acidification of freshwater is an environmental issue.[1]
Causes
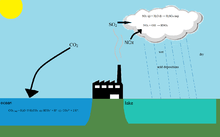
One of the causes of fresh water acidification is acid rain. That means rain that is unusually acidic (low pH). Acid rain has harmful effects on soil (soil acidification), animals, plants infrastructure and water.
Another cause of freshwater acidification is the Buffer solution. The soil usually has substances that ensure that the pH is neutral and that the acid will be removed: The Buffer solution. If the buffer solution is finished then the soil will become acid. This may cause toxic chemicals or nitrate to be released. The rain will cause the nitrate or the toxic chemicals to rinse out the surface water or ground water, causing them to contaminate water.
Harmful effects
Fish and other aquatic animals will die in water with low pH. The acidity of the water cannot be detected with the naked eye, the water may look clear and bright, but it may still be acid. Acidified water cannot be used for drinking. First the water has to be neutralized (neutral pH), as acidic water is damaging to your health and could possibly cause kidney problems.
Solution
Some countries try de-acidification of the lakes by adding a suspension of calcium carbonate. It is also possible to stop the environmental acidification by reducing the use of SO2 (Sulfur dioxide), [[NO<sub>x</sub>]] and NH3 (Ammonia). Lowering the use of these substances is done by using low-Sulphur fuel, or flue gas desulphurization.