Fibrodysplasia ossificans progressiva
| Fibrodysplasia ossificans progressiva | |
|---|---|
| Synonyms | Stoneman Disease |
 | |
| The effects of fibrodysplasia ossificans progressiva, a disease which causes damaged soft tissue to regrow as bone. | |
| Specialty | Medical genetics |
Fibrodysplasia ossificans progressiva (FOP) is an extremely rare connective tissue disease. The disease is caused by a mutation of the body's repair mechanism, which causes fibrous tissue (including muscle, tendon, and ligament) to be ossified spontaneously or when damaged. In many cases, injuries can cause joints to become permanently frozen in place. Surgical removal of the extra bone growths has been shown to cause the body to "repair" the affected area with even more bone.
Signs and symptoms
For unknown reasons, children born with FOP have deformed big toes, sometimes missing a joint or, in other cases, simply presenting with a notable lump at the minor joint. The first "flare-up" that leads to the formation of FOP bones usually occurs before the age of 10. The bone growth progresses from the top of the body downward, just as bones grow in fetuses. A child with FOP will typically develop bones starting at the neck, then on the shoulders, arms, chest area and finally on the feet.
Specifically, ossification is typically first seen in the dorsal, axial, cranial and proximal regions of the body. Later the disease progresses in the ventral, appendicular, caudal and distal regions of the body.[1] However, it does not necessarily occur in this order due to injury-caused flare-ups. Often, the tumor-like lumps that characterize the disease appear suddenly. This condition causes loss of mobility to affected joints, including the inability to fully open the mouth, limiting speech and eating; a specific occurrence of this condition to the foot joints can make an FOP patient unable to wear shoes without high heels as their feet can never be completely flat on the ground. Extra bone formation around the rib cage restricts the expansion of lungs and diaphragm causing respiratory complications.
Since the disease is so rare, the condition may be misdiagnosed as cancer or fibrosis. This leads physicians to order biopsies, which can exacerbate the growth of these lumps.[2] The presence of malformed toes or thumbs in those born with FOP help distinguish this disorder from other skeletal problems.[3]
The median age of survival is 40 years with proper management. However, delayed diagnosis, trauma and infections can decrease life expectancy.[4]
Causes
FOP is caused by an autosomal dominant allele on chromosome 2q23-24.[5] The allele has variable expressivity, but complete penetrance. Most cases are caused by spontaneous mutation in the gametes; most people with FOP cannot or choose not to have children. A similar but less catastrophic disease is fibrous dysplasia, which is caused by a post-zygotic mutation.
A mutation in the gene ACVR1 (also known as activin-like kinase 2 (ALK2)) is responsible for the disease.[6] ACVR1 encodes activin receptor type-1, a BMP type-1 receptor. The mutation causes substitution of codon 206 from arginine to histidine in the ACVR1 protein.[7][8] This substitution causes abnormal activation of ACVR1, leading to the transformation of connective tissue and muscle tissue into a secondary skeleton. This causes endothelial cells to transform to mesenchymal stem cells and then to bone.[9]
Genetics
FOP is an autosomal dominant disorder. Thus, children of an affected heterozygous parent and an unaffected parent have a 50% probability of being affected. Two affected individuals can produce unaffected children. The homozygous dominant form is more severe than the heterozygous form.[10]
The gene that causes ossification is normally deactivated after a fetus's bones are formed in the womb, but in patients with FOP, the gene keeps working. Aberrant bone formation in patients with FOP occurs when injured connective tissue or muscle cells at the sites of injury or growth incorrectly express an enzyme for bone repair during apoptosis (self-regulated cell death), resulting in lymphocytes containing excess bone morphogenetic protein 4 (BMP4) provided during the immune system response. The bone that results occurs independently of the normal skeleton, forming its own discrete skeletal elements. These elements, however, can fuse with normal skeletal bone.[11] The diaphragm, tongue, and extra-ocular muscles are spared in this process, as well as cardiac and smooth muscle.[1] Since the incorrect enzyme remains unresolved within the immune response, the body continues providing the incorrect BMP4-containing lymphocytes. BMP4 is a product that contributes to the development of the skeleton in the normal embryo.[12]
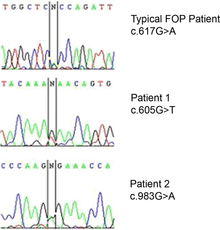
DNA sequencing electropherograms of a typical FOP patient can differ when being compared to two other patients. The ACVR1 gene encodes a bone morphogenic protein (BMP) receptor; this gene is mutated in FOP. This protein is responsible for growth and development of bone and muscles. Scientists theorize that a mutation in the ACVR1 changes the shape of the receptor and disrupts certain mechanisms that control the receptor's activity. There is a certain molecule, otherwise known as a ligand, that binds at the site to cause this reaction to activate with which it forms a complex. Due to the mutation, however, the bind site is modified and no longer stops the reaction.[13] The end result is an overgrowth of bone and cartilage and fusion of joints.[14]
Most of the cases of FOP were results of a new gene mutation: these people had no history of this particular disorder in their family. There are some cases which have shown people inheriting the mutation from one affected parent.[14]
Diagnosis
Outbreaks may be measurable clinically by elevated levels of alkaline phosphatase and bone-specific alkaline phosphatase.[15]
Treatment
There is no cure or approved treatment for FOP.[16] Attempts to surgically remove the bone may result in explosive bone growth.[17] While under anesthesia, people with FOP may encounter difficulties with intubation, restrictive pulmonary disease, and changes in the electrical conduction system of the heart.[18] Activities that increase the risk of falling or soft tissue injury should be avoided, as even minor trauma may provoke heterotopic bone formation.[19]
Epidemiology
As of 2017, approximately 800 cases of FOP have been confirmed worldwide making FOP one of the rarest diseases known.[16] The estimated incidence of FOP is 0.5 cases per million people and affects all ethnicities.[16]
History
Medical reports describing individuals affected by FOP date back as far as the seventeenth century.[16] FOP was originally called myositis ossificans progressiva and was thought to be caused by muscular inflammation (myositis) that caused bone formation.[16] The disease was renamed by Victor A. McKusick in 1970 following the discovery that soft tissue other than muscles (e.g. ligaments) were also affected by the disease process.[16]
The best known FOP case is that of Harry Eastlack (1933–1973). His condition began to develop at the age of ten, and by the time of his death from pneumonia in November 1973, six days before his 40th birthday, his body had completely ossified, leaving him able to move only his lips. Eastlack only lived to meet one other person with his same disease.
Eastlack donated his body to science. His skeleton is now at the Mütter Museum in Philadelphia, and has proven to be an invaluable source of information in the study of FOP.
Research
Clinical trials of isotretinoin, etidronate with oral corticosteroids, and perhexiline maleate have failed to demonstrate effectiveness, though the variable course of the disease and small prevalence induces uncertainty.[15]
A handful of pharmaceutical companies focused on rare disease are currently in varying stages of investigation into different therapeutic approaches for FOP.
In August 2015, U.S. Food and Drug Administration (FDA) Office of Orphan Products Development granted La Jolla Pharmaceuticals orphan drug designation for two novel compounds for FOP. The compounds are small-molecule kinase inhibitors designed to selectively block ACVR1 (ALK2).[20]
In August 2015, Clementia Pharmaceuticals also began the enrollment of children (ages 6 and above) into its Phase II clinical trial investigating palovarotene for the treatment of FOP.[21] Preclinical studies demonstrated that palovarotene, a retinoic acid receptor gamma agonist, blocked abnormal bone formation in animal models via inhibition of secondary messenger systems in the BMP pathway.[22] Clementia licensed palovarotene from Roche Pharmaceuticals, which previously evaluated the compound in more than 800 individuals including healthy volunteers and patients with chronic obstructive pulmonary disease. Palovarotene received Fast Track designation from the U.S. Food and Drug Administration (FDA) and orphan designations for the treatment of FOP from both the FDA and the European Medicines Agency (EMA).[21]
In September 2015, Regeneron announced new insight into the mechanism of disease involving the activation of the ACVR1 receptor by activin A. In 2016, the company initiated a phase 1 study of its activin antibody, REGN 2477, in healthy volunteers; a phase 2 trial in FOP patients was conducted in 2017.[23]
Another potential therapeutic approach involves allele-specific RNA interference that targets mutated mRNA for degradation while preserving normal ACVR1 gene expression.[24]
Further investigation into the mechanisms of heterotopic bone formation in FOP could aid in the development of treatments for other disorders involving extra-skeletal bone formation.
Fibro/adipogenic progenitors (FAPs) may be the disease causing cell type responsible for activin A dependent ectopic bone formation in both the muscles and tendons of mice bearing the FOP causing ACVR1(R206H) mutation.[25]
See also
- International FOP Association
- Osteogenesis imperfecta, a condition characterized by bones breaking unusually easily, caused by mutations in genes related to those responsible for FOP.
- FOP Friends
References
- 1 2 Fibrodysplasia ossificans progressiva. Frederick S. Kaplan, MD, Martine Le Merrer, MD, PhD, Professor of Genetics, David L. Glaser, MD, Robert J. Pignolo, MD, PhD, Robert Goldsby, MD, Joseph A. Kitterman, MD, Jay Groppe, PhD, and Eileen M. Shore, PhD
- ↑ Obamuyide HA, Ogunlade SO. A Tumour for which Surgery will do more harm than good: A Case Report of Fibrodysplasia Ossificans Progressiva. Niger Postgrad Med J. 2015;22(1):83-8.
- ↑ "Fibrodysplasia ossificans progressiva". Lister Hill National Center for Biomedical Communications. Retrieved 2013-12-12.
- ↑ "Fibrodysplasia ossificans progressiva with minor unilateral hallux anomaly in a sporadic case from Northern Tanzania with the common ACVR1c.617G>A mutation". The Pan African Medical Journal. 22. doi:10.11604/pamj.2015.22.299.8032.
- ↑ Feldman, G. "A recurrent mutation in the BMP type I receptor ACVR1 ( HSC'13) causes inherited and sporadic fibrodysplasia ossificans progressiva" (PDF).
- ↑ Shore EM; Xu M; Feldman GJ; et al. (2006). "A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva". Nat. Genet. 38 (5): 525–527. doi:10.1038/ng1783. PMID 16642017.
- ↑ News Release of FOP's Cause Archived 2012-01-13 at the Wayback Machine.
- ↑ "ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A". stm.sciencemag.org.
- ↑ Dinther; et al. (2010). "ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation". Journal of Bone and Mineral Research. 25: 091211115834058–35. doi:10.1359/jbmr.091110. PMID 19929436.
- ↑ Cummings, Michael R. "Human Heredity: Principles and Issues". Cengage Learning, 2011, 2009. p.77
- ↑ Shore Eileen M.; Kaplan Frederick S. (2008). "Insights from a Rare Genetic Disorder of Extra-Skeletal Bone Formation, Fibrodysplasia Ossificans Progressiva (FOP)". Bone. 43 (3): 427–43. doi:10.1016/j.bone.2008.05.013. PMC 2601573. PMID 18590993.
- ↑ Kierszenbaum, Abraham (2002). Histology and cell biology. New York: Mosby. ISBN 978-0-323-01639-1.
- ↑ "AVCR1",Genetics Home Reference, U.S. National Library of Medicine, August 2007. Accessed February 18, 2014.
- 1 2 "Fibrodysplasia ossificans progressiva",Genetics Home Reference, U.S. National Library of Medicine, August 2007. Accessed February 18, 2014.
- 1 2 Hiroshi Kitoh; et al. (2013). "Perhexiline maleate in the treatment of fibrodysplasia ossificans progressiva: an open-labeled clinical trial". Orphanet Journal of Rare Diseases. 8: 163. doi:10.1186/1750-1172-8-163.
- 1 2 3 4 5 6 Martelli A, Santos AR Jr (2014). "Cellular and morphological aspects of fibrodysplasia ossificans progressiva. Lessons of formation, repair, and bone bioengineering". Organogenesis (Review). 10 (3): 303–11. doi:10.4161/org.29206. PMC 4750545. PMID 25482313.
- ↑ American Academy of Orthopaedic Surgeons (May 2006). "Fibrodysplasia Ossificans Progressiva (FOP)". orthoinfo.aaos.org. Archived from the original on 21 June 2012. Retrieved 2011-10-07.
- ↑ Newton, M.C.; Allen, P.W.; Ryan, D.C. "Fibrodysplasia Ossificans Progressiva". British Journal of Anaesthesia. Retrieved October 25, 2011.
- ↑ http://www.ifopa.org/treatment-guidelines.html
- ↑ "La Jolla Pharmaceutical Company Receives Orphan Drug Designation for Two Novel Compounds for Fibrodysplasia Ossificans Progressiva". Business Wire. August 18, 2015. Retrieved 22 November 2015.
- 1 2 "Clementia Pharmaceuticals Expands Ongoing Phase 2 Study to Include Children with Fibrodysplasia Ossificans Progressiva (FOP)". PRNewswire. August 25, 2015. Retrieved 22 November 2015.
- ↑ "Pipeline". www.clementiapharma.com. Archived from the original on 29 September 2015. Retrieved 22 November 2015.
- ↑ "Regeneron Shares Updates on its Ongoing FOP Research Program". International Fibrodysplasia Ossificans Progressiva Association. March 9, 2017.
- ↑ J. W. Lowery; V. Rosen (2012). "Allele-Specific RNA Interference in FOP Silencing the FOP gene". Gene Therapy. 19 (701–702): 701–702. doi:10.1038/gt.2011.190.
- ↑ John B. Lees-Shepard; Masakazu Yamamoto; Arpita A. Biswas; et. al (2018). "Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva". Nature Communications. 9 (471). doi:10.1038/s41467-018-02872-2.
Further reading
- Cohen, MM; Howell, RE (October 1999). "Etiology of fibrous dysplasia and McCune-Albright syndrome". International journal of oral and maxillofacial surgery. 28 (5): 366–71. doi:10.1016/s0901-5027(99)80085-x. PMID 10535539.
External links
| Classification | |
|---|---|
| External resources |