Fialuridine
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | 2′-Fluoro-5-iodouracil |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| Chemical and physical data | |
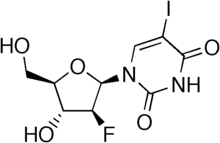
| Formula | C9H10FIN2O5 |
| Molar mass | 372.09 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Fialuridine, or 1-(2-deoxy-2-fluoro-1-D-arabinofuranosyl)-5-iodouracil (FIAU), is a nucleoside analogue that was investigated as a potential therapy for hepatitis B virus infection. In a 1993 clinical study at the NIH, unexpected toxicity led to the death of 5 out of 15 patients from liver failure associated with lactic acidosis; two further participants required liver transplantation. This toxicity was unusual in that it was not predicted by animal studies. It is suspected that the drug's toxicity was due to its damaging mitochondria.[1][2][3]
References
- ↑ Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol. 2011 Apr;8(4):202-11. Review. PMID 21386809
- ↑ McKenzie R, Fried MW, Sallie R, et al. (1995). "Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B". N. Engl. J. Med. 333 (17): 1099–1105. doi:10.1056/NEJM199510263331702. PMID 7565947.
- ↑ Thomson, Larry (1 March 1994). "The Cure that Killed". Discover. Retrieved 2 November 2013.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.